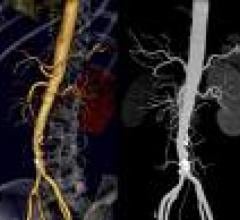
Fractional flow reserve (FFR) is starting to change cardiologists’ minds about how to treat patients since the ...
Cath Lab
The catheterization lab channel includes content related to interventional technologies for coronary and peripheral artery disease (PAD). Other cath lab transcatheter device technologies covered on this page included percutaneous treatment for stroke, venous interventions, heart valves, hypertension, heart failure and percutenous coronary interventions (PCI).
February 9, 2010 - The U.S. Food and Drug Administration says it is starting a program designed to reduce ...
February 9, 2010 – The FDA approved CRESTOR (rosuvastatin calcium) to reduce the risk of stroke, myocardial ...
Adjustable parameters on the ACIST® CVI® Contrast Delivery System include, flow rate, volume, pressure limit, rise time ...
At the American College of Cardiology (ACC) 2010, Philips Healthcare will focus on the full spectrum of cardiology ...
For more than a decade, Barry T. Katzen, M.D., medical director of Baptist Cardiac and Vascular Institute (BCVI) ...
February 3, 2010 – The new Gator ClipSeal Plug is designed to maintain a hemostatic seal around 0.035- or 0.038 ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...
February 2, 2010 - A second-generation transradial compression bracelet, the Zoom 2010, is being released by Zoom ...
The ACCULINK carotid stent is designed to provide easy and accurate stent placement in patients who have carotid ...
The ACCUNET embolic protection system is designed to provide excellent capture capabilities and easy filter ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
February 2, 2010 – Data published in EuroIntervention (EuroIntervention, 2010; 5:698-702) demonstrate good safety ...
February 1, 2010 – The FDA granted market clearance for the PICC WAND introducer catheter, which enables ...
February 1, 2010 – Three patent disputes between Boston Scientific Corp. and Johnson & Johnson (J&J) were settled ...
During cardiology fellowship, my institution had a hand manifold system. I found it difficult to use at times, and it ...
January 27, 2010 – New 6, 7 and 8 mm diameters of the Maris Plus self-expanding peripheral stent system, were ...
January 25, 2010 – Expanding its peripheral vascular disease product offerings, Medtronic Inc. today signed an ...
January 25, 2010 – Trials for a stent that attracts endothelial progenitor cells show positive results in ...


 February 10, 2010
February 10, 2010