October 8, 2009 – Boston Scientific Corp. this week announced its exclusive sponsorship and first enrollments of ...
Catheters
October 7, 2009 – FlowCardia Inc. this week launched the FlowMate Injector, which dramatically simplifies central ...
September 11, 2009 – Terumo Interventional Systems yesterday launched its Glidewire Advantage Peripheral Guidewire ...
August 3, 2009 – Abbott is conducting a voluntary recall of its POWERSAIL Coronary Dilatation Catheters, which may ...
July 28, 2009 – Onset Medical Corporation said today it received CE mark approval to begin marketing the SoloPath ...

Interventionalists using radial artery access instead of femoral access say it significantly cuts bleeding complications ...
July 17, 2009 – Medtronic announced today the completion of a 12-month follow-up in the STOP-AF (Sustained ...
July 8, 2009 – CardioFocus Inc. said yesterday it received a CE mark in Europe for its Endoscopic Ablation System ...
July 8, 2009 – A procedure that sends targeted energy into the heart through a catheter can be used to treat a ...
June 15, 2009 - Inovio Biomedical Corp. said today it granted an option to a commercial license to develop ...
May 19, 2009 - Volcano Corp. yesterday announced an exclusive worldwide distribution agreement to initiate the ...
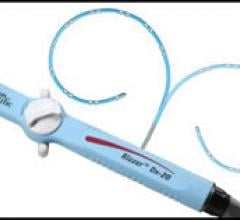
May 18, 2009 – Boston Scientific showcased its new Blazer Dx-20, a bidirectional duodecapolar diagnostic catheter ...
May 14, 2009 – In final, one-year results of a multicenter randomized clinical study, catheter ablation ...
May 13, 2009 – Siemens Healthcare is demonstrating the clinical benefits of the world’s first volume intracardiac ...
May 6, 2009 - Spectranetics Corp. yesterday said it made a pre-IDE (investigational device exemption) submission ...


 October 08, 2009
October 08, 2009