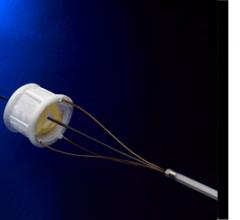
July 16, 2010 – Biosense Webster Inc. recently announced that more than 10 patients have now been enrolled in the ...
Catheters
June 24, 2010 – The National Institutes of Health (NIH) announced this week that it has given a $2.2 million grant ...
June 21, 2010 – Long-term data presented today from Ardian’s Symplicity HTN-1 study demonstrate that the ...
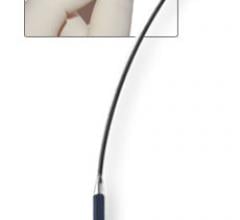
June 10, 2010 – The single-use EZ Steer bi-directional catheters are now cleared for reprocessing through Ascent ...
May 20, 2010 – A fractional flow reserve (FFR) guide wire gained U.S. Food and Drug Administration (FDA) ...
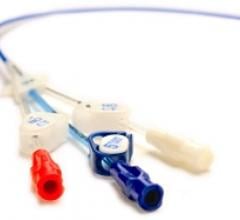
May 19, 2010 – Three distinct lumens in a new line of peripherally inserted central catheters (PICCs) increase ...
April 22, 2010 – Instead of using a conventional spring coil design, a new guidewire uses a micro-cut nitinol ...
April 13, 2010 – A catheter designed to deliver embolic materials and radiopaque media to selected sites in ...
The GuideLiner catheter is a coaxial “mother and child” guide extension using a rapid exchange system that ...
A new and improved design of the original Gopher catheter, the Gopher Gold is designed for use when treating ...
February 1, 2010 – The FDA granted market clearance for the PICC WAND introducer catheter, which enables ...
January 15, 2010 – A Dutch multicenter trial (DUET) is comparing ultrasound (US) accelerated catheter-directed ...
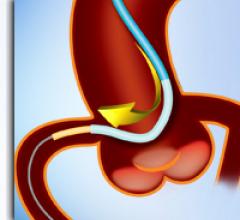
November 24, 2009 – European CE mark clearance was granted this week for BridgePoint Medical’s coronary and ...
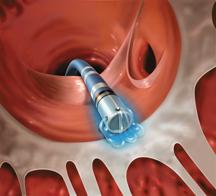
The incidence of cardiac arrhythmia is expected to increase significantly with an aging U.S. population and rising ...
November 10, 2009 – Onset Medical Corp. today said it received U.S. FDA 510(k) marketing clearance for its ...

 July 15, 2010
July 15, 2010