December 12, 2013 — BioCardia Inc., a cardiovascular regenerative medicine company, announced positive 12-month results ...
Diagnostic and Interventional Cardiology DAIC
Diagnostic and Interventional Cardiology (DAIC) is a magazine that reaches more than 25,000 healthcare professionals in cardiology, interventional cardiology and cath labs across the United States. These influential buying team members rely on DAIC's award-winning editorial content and comparison charts as a unique research tool for specifying, recommending and approving technology/device purchases.
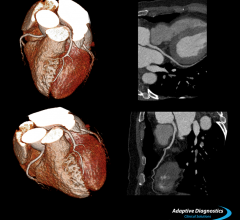
December 12, 2013 — Toshiba introduced its Adaptive Diagnostics computed tomography (CT) technology, which simplifies ...
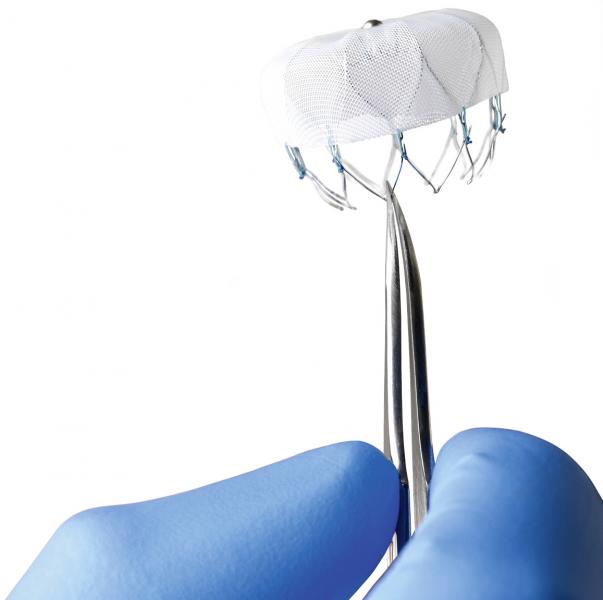
December 12, 2013 — The U.S. Food and Drug Administration (FDA) Circulatory System Devices Panel of the Medical Devices ...
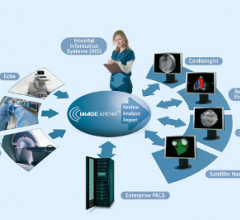
December 11, 2013 — Novarad’s NovaCardio electrocardiogram (ECG) integrates with cardiology picture archiving and ...
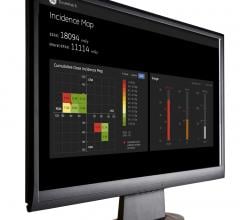
December 11, 2013 – The National Cheng Kung University Heart Science and Medical Devices Research Center (NCKU HSDMRC) ...
December 10, 2013 — TomTec Imaging Systems GmbH announced the U.S. Food and Drug Administration (FDA) 510(k) clearance ...
December 10, 2013 — GE Healthcare showcased several new offerings that enable low-dose imaging procedures and help ...
December 10, 2013 – Konica Minolta Medical Imaging announced that its parent company, Konica Minolta, Inc., has signed a ...

December 9, 2013 — Seroj Avoyan is a world class Armenian Dhol drum virtuoso whose fingers move with such dexterity they ...
December 9, 2013 — With increased penalties in effect for hospitals with excessive readmissions for heart attack and hea ...
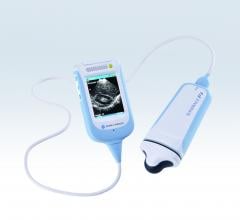
December 9, 2013 — Konica Minolta Medical Imaging announced the launch of the Sonimage P3 hand-held ultrasound device ...
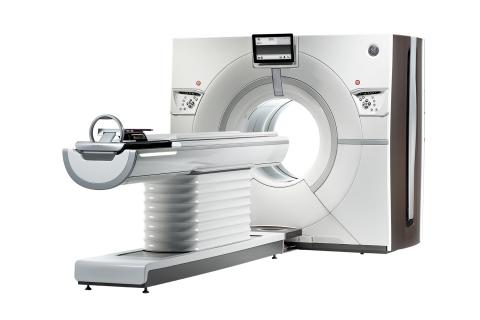
December 9, 2013 — GE Healthcare announced at the Radiological Society of North America Annual Meeting (RSNA 2013) in ...
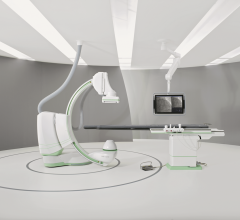
December 6, 2013 — Siemens Healthcare introduced an angiography system optimized for broad clinical utilization at the R ...
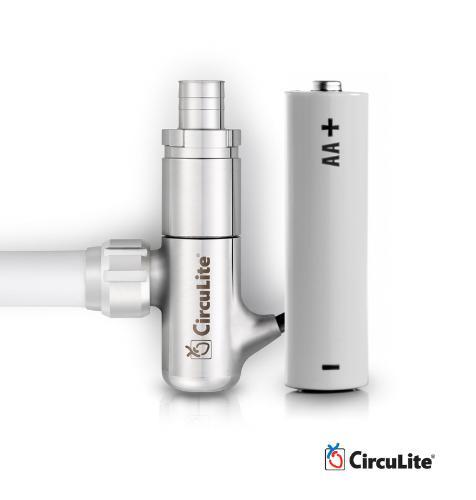
December 6, 2013 — HeartWare International Inc. has acquired CircuLite Inc., a developer of the Synergy Circulatory ...

December 6, 2013 — Medtronic Inc. announced CE mark in Europe and Therapeutic Goods Administration (TGA) listing in ...

 December 12, 2013
December 12, 2013