January 24, 2014 — Boston Scientific has received U.S. Food and Drug Administration (FDA) clearance and CE mark approval ...
Diagnostic and Interventional Cardiology DAIC
Diagnostic and Interventional Cardiology (DAIC) is a magazine that reaches more than 25,000 healthcare professionals in cardiology, interventional cardiology and cath labs across the United States. These influential buying team members rely on DAIC's award-winning editorial content and comparison charts as a unique research tool for specifying, recommending and approving technology/device purchases.
January 23, 2014 – Men with enlarged prostates can find relief with a non-surgical treatment that shrinks the gland ...
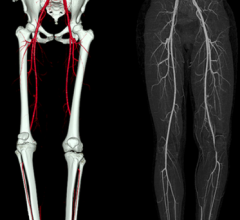
January 24, 2014 — Peripheral artery disease (PAD) sufferers maintain improved quality of life, including being able to ...
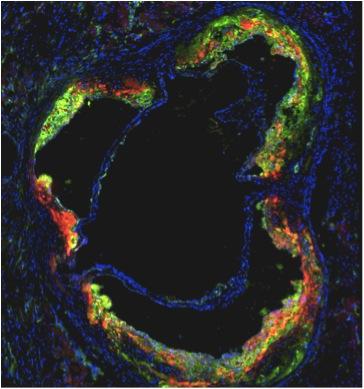
January 23, 2014 — Up to 30 percent of heart attack patients suffer an additional heart attack because of limited ...
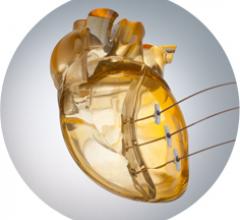
January 22, 2014 — BioVentrix’s Revivent-TC Ventricular Enhancement System was implanted in a man via Less Invasive ...
January 22, 2014 — BeneChill Inc. received the 2014 Frost & Sullivan Award for Technology Innovation Leadership for its ...
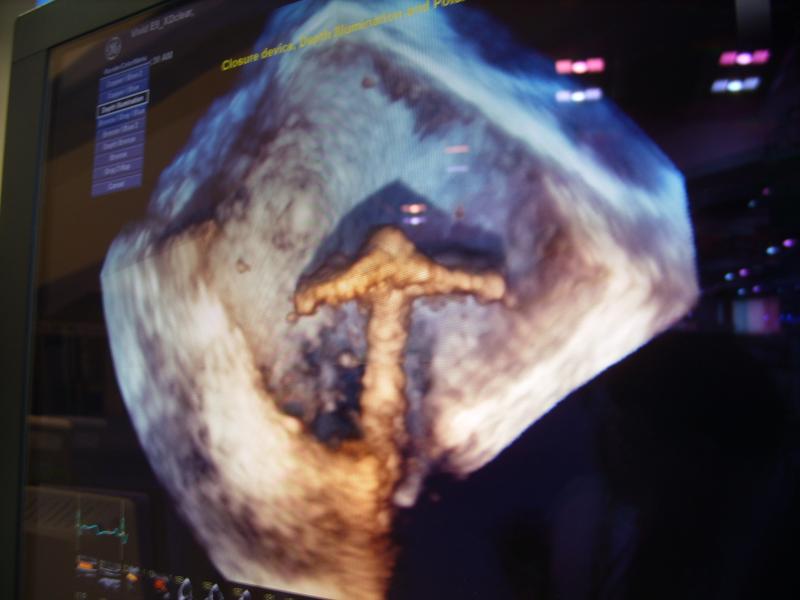
Feature | Dave Fornell
The Cardiovascular Research Foundation’s Transcatheter Cardiovascular Therapeutics (TCT) 2013 offers many new insights ...
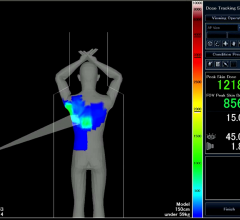
January 20, 2014 — Toshiba America Medical Systems’ Dose Tracking System allows clinicians to track X-ray skin dose ...
Technology | Dave Fornell
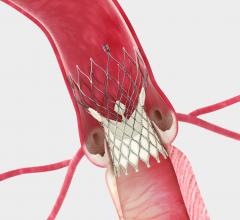
January 17, 2014 — The U.S. Food and Drug Administration (FDA) granted U.S. market approval for Medtronic’s self ...

Feature | Dave Fornell
In a move that calls into question the future of transcatheter valve competition in the United States, a jury in the ...

January 14, 2014 — Edwards Lifesciences Corp. announced it received investigational device exemption (IDE) approval from ...
January 14, 2014 — MediValve Ltd. announced that it has received clearance of a Pre-Marketing Notification Application ...
January 10, 2014 — CVRx Inc. announced findings from a health-economic analysis published in the Journal of Hypertension ...
January 9, 2014 — Highly invasive therapies are challenging due to the inability to quickly and safely secure devices ...
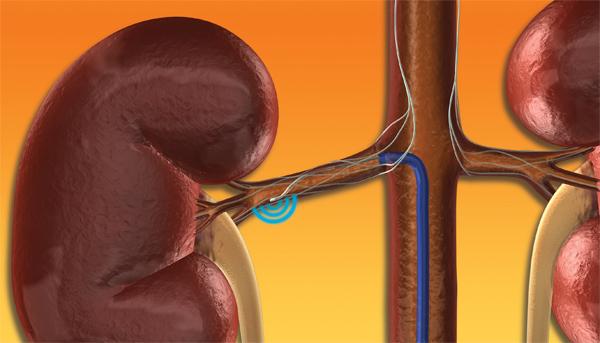
January 9, 2014 — Medtronic Inc. announced its U.S. pivotal trial for its Symplicity renal denervation system to treat ...

 January 24, 2014
January 24, 2014