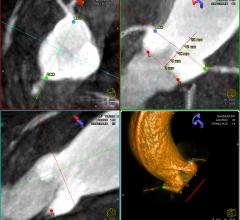
February 24, 2012 — Simbionix USA Corp. announced that it received U.S. Food and Drug Administration (FDA) clearance for ...
Diagnostic and Interventional Cardiology DAIC
Diagnostic and Interventional Cardiology (DAIC) is a magazine that reaches more than 25,000 healthcare professionals in cardiology, interventional cardiology and cath labs across the United States. These influential buying team members rely on DAIC's award-winning editorial content and comparison charts as a unique research tool for specifying, recommending and approving technology/device purchases.
Teleflex Inc. announced a new agreement with HealthTrust Purchasing Group L.P. (HealthTrust), for its Arrow intra-aortic ...
February 22, 2012 — HeartWare International Inc. announced that a U.S. Food and Drug Administration (FDA) Circulatory ...
February 20, 2012 — ReCor Medical announced that its Paradise percutaneous ultrasound renal denervation system for ...
February 20, 2012 — Edwards Lifesciences Corp. announced that it received CE mark in Europe for its Edwards Intuity ...
February 20, 2012 — Biopharmaceutical company Celladon Corp. this week completed a $43 million equity financing to ...
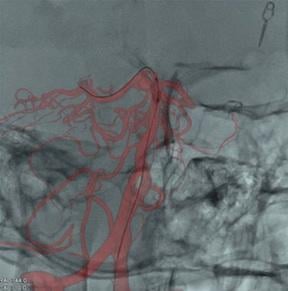
As catheter-based, minimally invasive procedures expand rapidly beyond treatment of the coronary arteries into all areas ...
February 16, 2012 — U.S. Food and Drug Administration (FDA) granted 510(k) clearance for AngioDynamics NeverTouch Direct ...
February 16, 2012 — Few hospitals have as much experience with the Berlin Heart pediatric ventricular assist device (VAD ...
February 16, 2012 — Siemens Healthcare received clearance from the U.S. Food and Drug Administration (FDA) for syngo Aor ...
February 16, 2012 — Velomedix Inc., a clinical stage medical device company, received investigational device exemption ...
February 16, 2012 — Aptus Endosystems Inc. a medical device company developing advanced technology for endovascular ...
February 15, 2012 — Vessix Vascular Inc., developer of a novel percutaneous radiofrequency (RF) balloon catheter ...
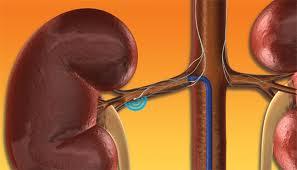
February 15, 2012 — Medtronic Inc. announced the start of two clinical initiatives evaluating the broader, real-world ...
Being a child of the late 1970s and 1980s, I was a big Star Wars fan. Somewhere in my basement I still have the action ...

 February 24, 2012
February 24, 2012