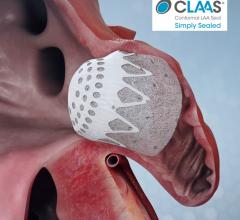
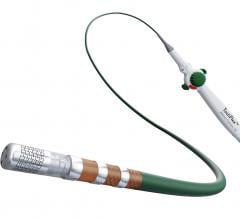
September 8, 2020 - A new study presented at the 2020 European Society of Cardiology (ESC) Congress shows that Biotronik ...
EP Lab
This channel includes news and new technology innovations for cardiac electrophysiology (EP) systems, techniques and devices using in EP labs. This includes implantable EP devices, pacemakers, implantable cardioverter defibrillators (ICD), cardiac resychronization therapy (CRT), ablation technologies, left atrial appendage (LAA) occlusion, atrial fibrilation (AF) and Holter monitors.
September 8, 2020 — Researchers developed a new class of medical instruments equipped with an advanced soft electronics ...
September 3, 2020 – Biosense Webster launched its Carto 3 System Version 7 and the Carto Prime Mapping Module to enhance ...
When the patients of Michael Boler, M.D. need cardiac monitoring, the Holter monitor is no longer his first choice. “The ...
August 21, 2020 — According to a report published by Research Dive, the rising number of patients suffering from atrial ...
August 17, 2020 — A Northwestern Medicine Bluhm Cardiovascular Institute physician was the first electrophyiologist in ...
All medical conferences moved to a virtual meeting format due to COVID-19 this year, and there has been apprehension ...
Apple created a stir when it announced in 2018 that its Apple Watch Series 4 was the first consumer health and fitness ...
August 6, 2020 – Conformal Medical Inc. announced it secured $85 million in Series C financing to support the company’s ...
August 4, 2020 — Abbott announced the first enrollments in the TactiFlex PAF investigational device exemption (IDE) ...
Devi G. Nair, M.D., FHRS, director of cardiac electrophysiology, St. Bernards Heart and Vascular Center, Jonesboro, Ark ...
Washington Health System (WHS) provides healthcare services at more than 40 offsite locations across three counties in ...
Devi G. Nair, M.D., FHRS, director of cardiac electrophysiology, St. Bernards Heart and Vascular Center, Jonesboro, Ark ...
July 22, 2020 — The U.S. Food and Drug Administration (FDA) has approved Boston Scientific's next generation Watchman ...
July 13, 2020 - The U.S. Food and Drug Administration (FDA) has approved Abbott's next-generation Gallant implantable ...
Apple created a stir when it announced in 2018 that its Apple Watch Series 4 was the first consumer health and fitness ...
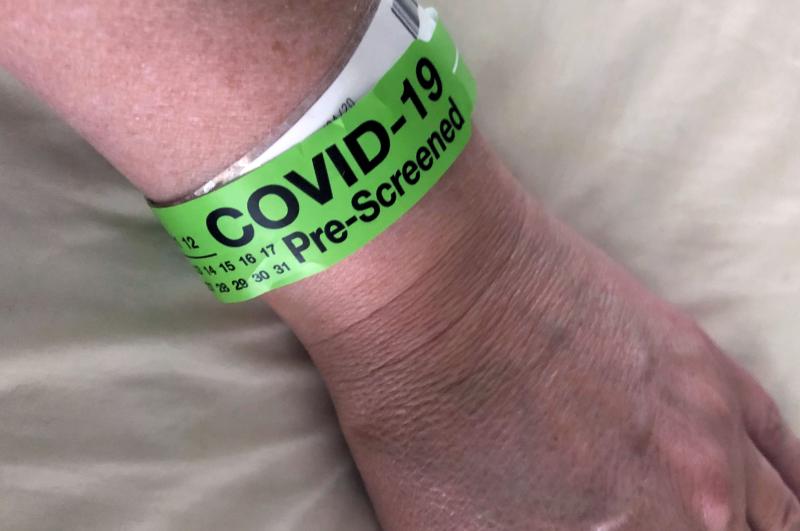
July 9, 2020 — Older, critically ill patients with COVID-19 (SARS-CoV-2) who received a combination of the ...
July 7, 2020 – Medtronic announced it received U.S. Food and Drug Administration (FDA) clearance and European CE mark ...
June 29, 2020 — Boston Scientific received U.S. Food and Drug Administration (FDA) 510(k) clearance for the LUX-Dx Inser ...


 September 08, 2020
September 08, 2020