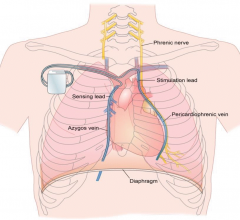
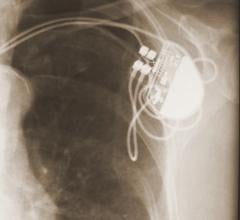
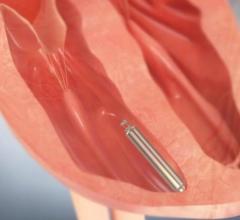
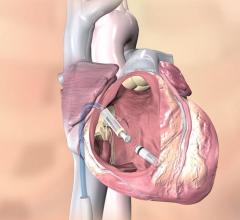
November 10, 2017 — Mexican doctors have safely reused donated pacemakers after sterilization, according to a study ...
Pacemakers
This channel includes news and new technology innovations for pacemakers used to treat bradycardia.
October 18, 2017 — The U.S. Food and Drug Administration (FDA) recently approved a new treatment option for patients who ...
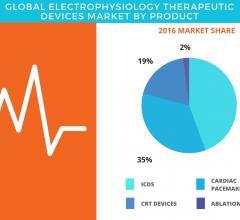
September 11, 2017 — According to the latest market study released by Technavio, the global electrophysiology ...

August 29, 2017 — The U.S. Food and Drug Administration (FDA) approved a firmware update that is now available to reduce ...
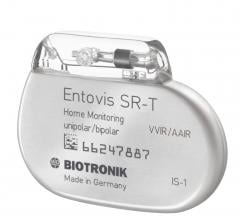
August 21, 2017 — Biotronik announced U.S. Food and Drug Administration (FDA) approval and commercial availability of ...
July 27, 2017 — There is good news when it comes to the heart’s sinoatrial node (SAN), the body’s natural pacemaker ...
July 3, 2017 — Medtronic recently announced that its Reactive ATP therapy slows the progression of atrial fibrillation ...

Electrophysiology (EP) technology has been advancing rapidly the past few years with new ablation tools to improve ...
June 20 2017 — Pacemakers and other cardiac devices can help solve forensic cases, according to a study presented at the ...
DAIC Editor Dave Fornell takes a tour of some of the most innovative new electrophysiology (EP) technology at the 2017 H ...
Bruce Wilkoff, M.D., director of cardiac pacing and tachyarrhythmia devices at Cleveland Clinic, discusses advancements ...
Vivek Reddy, M.D., director of cardiac arrhythmia services and professor of medicine, cardiology, Mount Sinai Hospital ...

May 16, 2017 - The preliminary results for the Medtronic Micra Transcatheter Pacing System (TPS) Post-Approval Registry ...
This video, provided by Medtronic, demonstrates the implantation of Micra transcatheter pacing system (TPS). The device ...
A discussion with Heart Rhythm Society (HRS) President Michael Gold, M.D., Ph.D., director of cardiology and associate ...


 November 10, 2017
November 10, 2017