A major disappointment earlier this year was the announcement by Medtronic that its renal denervation system failed to ...
Renal Denervation
This channel includes news and new technology innovations for renal denervation used to treat hypertension. Renal denervation is a catheter-based therapy. The catheter is into the renal artery and delivers radio-frequency energy to ablate the nerves in the vessel wall that regulate vasoconstriction. This leaves the vessel permanently in the widest open position to allow greater blood flow to the kidneys. This enables greater filtration of the blood and removal of excess water that causes hypertension.
March 27, 2014 — Scientists at CBSET, a not-for-profit preclinical research institute dedicated to biomedical research ...
By Dave Fornell, DAIC editor
Data from several cutting-edge device clinical trials will be presented as late-breakers ...
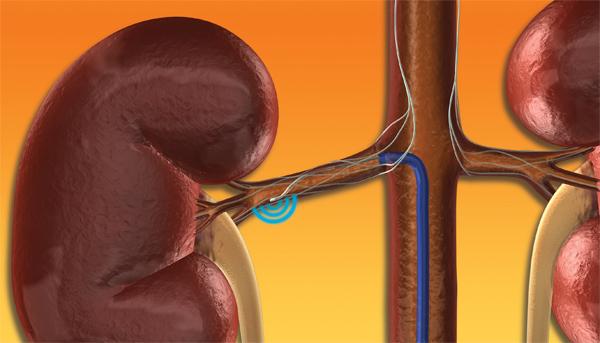

December 6, 2013 – Medtronic, Inc. presented three-year data from SYMPLICITY HTN-2, the first and longest-running ...
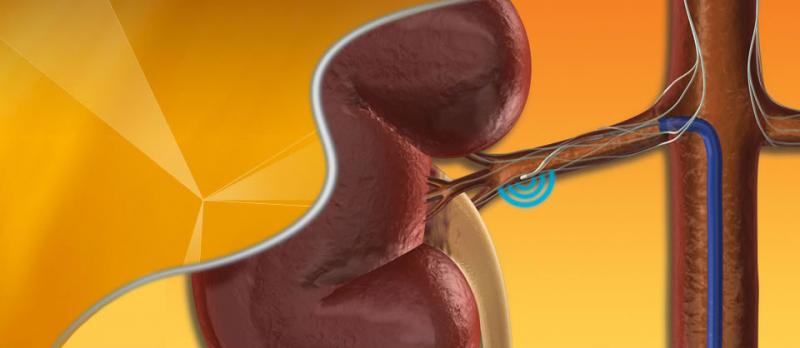


 April 03, 2014
April 03, 2014





