Here are the top 25 best performing articles on the Diagnostic and Interventional Cardiology (DAIC) website from 2020 ...
Structural Heart
This structural heart channel includes news, videos, podcasts and other content related to diagnosis and treatment of structural heart disease. Topics covered include heart valve repair and replacement, transcatheter aortic valve replacement (TAVR), transcatheter mitral valve replacement (TMVR), transcatheter tricuspid valve replacement (TTVR), left atrial appendage (LAA) occlusion, heart failure interventional device therapies, and closing holes in the heart using, including occlusion of atrial septal defects (ASDs), ventricular septal defects (VSDs) and patent foramen ovales (PFOs).
Here are the top performing 25 videos on the DAIC website in 2020. The picks are based on Google Analytics of the DAIC ...
December 14, 2020 - Foldax Inc. today announced that the U.S. Food and Drug Administration (FDA) has granted an ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...
December 1, 2020 — Here is the list of the most popular content on the Diagnostic and Interventional Cardiology (DAIC) ...
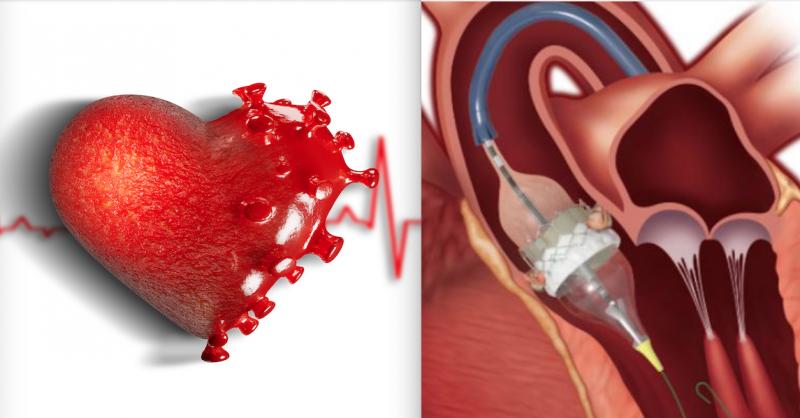
December 1, 2020 — A recent publication demonstrated procedural efficiency for MitraClip transcatheter mitral valve ...
November 18, 2020 — The primary results from the RIVER Trial, Rivaroxaban for Valvular Heart disease and Atrial ...
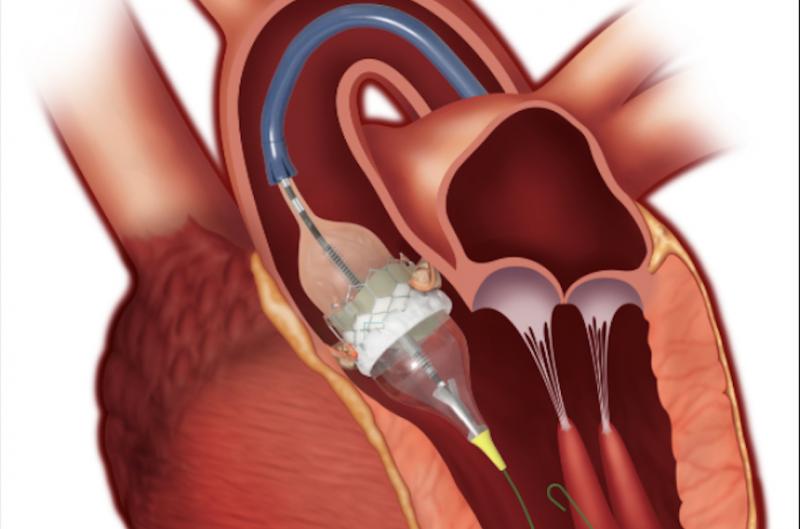
November 17, 2020 — Since the approval of the first transcatheter aortic valve replacement (TAVR) device in 2011, more ...

November 17, 2020 — Boston Scientific Corp. announced today it is immediately retiring the entire Lotus Edge ...
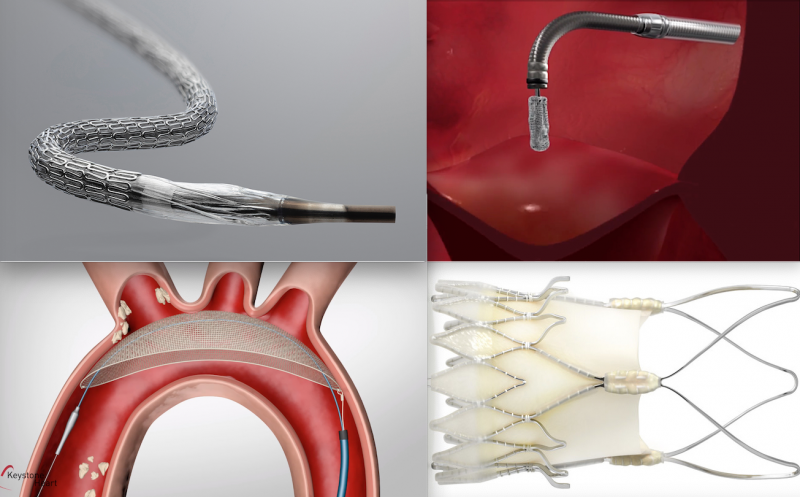
Here are some of the key takeaways from the late-breaking interventional cardiology and structural heart trials ...
Oct. 29, 2020 — Boston Scientific initiated the CHAMPION-AF clinical trial to evaluate the safety and efficacy of the ...
David Cohen, M.D., presents late-breaking data from the STS/ACC Transcatheter Valve Registry (TVT) showing the impact of ...
Doctor Hans-Josef Feistritzerm, Heart Center of Leipzig, Germany, presents data on the use of general vs. local ...
The late-breaking MitraBridge Study was presented at Transcatheter Cardiovascular Therapeutics (TCT) 2020 meeting showed ...
October 16, 2020 — Positive clinical data from first-in-human studies of the Conformal Medical left atrial appendage ...
October 15, 2020 – The REFLECT II randomized clinical trial evaluating the safety and efficacy of the Keystone Heart ...

 December 28, 2020
December 28, 2020