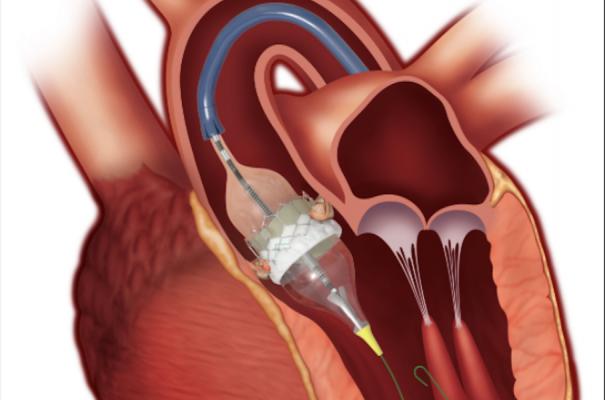
June 8, 2020 — Edwards Lifesciences Corp. announced Chinese regulatory approval for the Edwards Sapien 3 transcatheter aortic valve replacement (TAVR) system for the treatment of patients suffering from severe, symptomatic aortic stenosis (AS) at high risk for or unable to undergo open-heart surgery.
"Now, in China, patients diagnosed with severe AS have the option of a shorter procedure with excellent clinical outcomes, including a more rapid recovery than open-heart surgery," said Prof. Junbo Ge, M.D., academician of the Chinese Academy of Sciences, chairman, Shanghai Institute of Cardiovascular Diseases and director, Department of Cardiology, Zhongshan Hospital, Fudan University.
The Sapien 3 valve builds on Edwards' decades of experience in the development of tissue heart valves, and the proven benefits of the Edwards Sapien transcatheter heart valves. The Sapien TAVR valves are the most widely studied transcatheter valves, with more than 30,000 patients treated in clinical trials and registries in over 65 countries around the world. The approval for high-risk and extreme-risk patients in China was supported by the China Sapien 3 study, which complements a highly robust set of clinical outcomes from three randomized controlled PARTNER studies, along with excellent real-world results.
"This approval marks a major milestone for Chinese physicians and their patients living with severe AS in need of alternatives to open-heart surgery," said Larry L. Wood, Edwards' corporate vice president, TAVR. "Edwards is proud to introduce this technology, which has demonstrated successful outcomes for patients worldwide, into China. We look forward to partnering with hospitals throughout China to introduce this therapy through our comprehensive, globally proven training program."
The valve, available in 20, 23, 26 and 29 mm sizes, is approved by the National Medical Products Administration for treating high-risk patients in China. To date, more than 650,000 patients around the world have benefitted from TAVR.
For more information: www.Edwards.com
Related Sapien 3 TAVR Content:
TAVR Equivalent to Surgery at Two Years in Low-Risk Patients
Key TAVR Takeaways From ACC 2020
VIDEO: TAVR Performs as Well as Surgery in Low-Risk Patients — Interview with Michael Mack, M.D.
TAVR Equivalent to Surgery at Two Years in Low-Risk Patients
Sapien 3 Valve Shows Favorable One-Year Results in PARTNER II Trial


 November 14, 2025
November 14, 2025 









