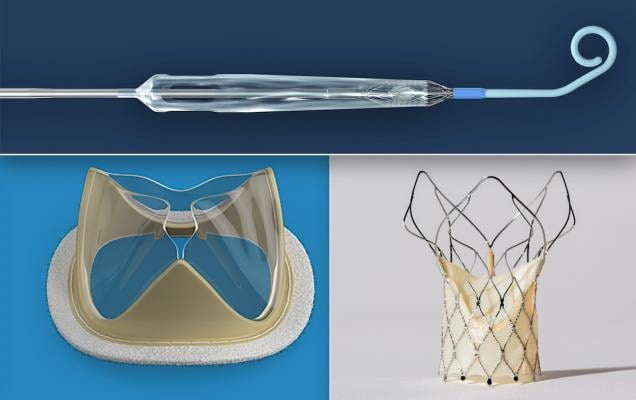
Three of the most popular cardiovascular technologies to make the top news in June 2020. Top is the Impella Expandable Cardiac Pump (ECP) that is about to enter U.S. feasibility trials.Bottom left, the Foldax heart valve uses a flexible man-made material for valve leaflets rather than animal pericardium tissue. The hope is this new type of surgical and transcatheter aortic valve replacements (TAVR) device will enhance longevity of the valve leaflets. Bottom right, the SMT Hydra TAVR valve is the first Indian-made structural heart devices to gain European CE mark approval.
July 6, 2020 — Here is the list of the most popular content on the Diagnostic and Interventional Cardiology (DAIC) magazine website from the month of June 2020. This is based on the website’s 267,198 pageviews for the month. While COVID-19 topics still are among the popular topics on the list, there has been a noticable return to normal reader interest in new cardiovascular technologies.
1. Kawasaki-like Inflammatory Disease Affects Children With COVID-19
2. The Cardiovascular Impact of COVID-19
3. FDA Approves First-in-Human Trial of Impella ECP, World’s Smallest Heart Pump
4. New Technologies Take Cardiac CT to the Next Level
5. SMT Hydra TAVR System Receives European CE Mark Approval
6. Foldax Gets $20 Million to Finance Innovative Heart Valve Technology
7. ASE Guidelines for the Protection of Echocardiography Providers During the COVID-19 Outbreak
8. Impella RP Granted FDA Emergency Use Authorization for COVID-19 Patients With Right Heart Failure
9. FDA Revokes Emergency Use Authorization for Chloroquine and Hydroxychloroquine for COVID-19
10. First Implantation of Carmat Total Artificial Heart in Denmark
11. Pulsed Field Ablation Successfully Treats Atrial Fibrillation
12. FDA Grants Breakthrough Device Designation for preCardia Catheter-based Heart Failure Treatment
13. New Heart Failure Devices and Drugs to Treat Heart Failure
15. New Guideline Outlines Use of Transesophageal Echo to Assist Surgical Decision-making
16. Use of Ultrasound to Improve Vascular Access
17. VIDEO: Demonstration of Abiomed Impella ECP 9 French Transcatheter Ventricular Assist Device
18. FDA Clears Scoring Sheath That Fits Over Standard Angioplasty Balloons
19. VIDEO: Top New EP Technologies at Heart Rhythm Society 2020
21. Edwards Pascal Tricuspid Valve Transcatheter Repair System Approved in Europe
22. Biotronik Launches His-Bundle Pacing Tools
23. Heart Rhythm Society 2020 Late-Breaking Clinical Trials in Electrophysiology
24. Cardiac Imaging Best Practices During the COVID-19 Pandemic
25. COVID-19 Genetic PCR Tests Give False Negative Results if Used Too Early
Related Popular Cardiology Content:
Most Popular Cardiology Technology Content in May 2020
Most Popular Cardiology Technology Content in March 2020
Most Popular Cardiology Technology Content in February 2020
Most Popular Cardiology Technology Content in January 2020
Top 25 Cardiology Videos on DAIC in 2019


 December 24, 2025
December 24, 2025 









