March 17, 2015 — The American College of Cardiology (ACC) hosted its 64th Annual Scientific Session & Expo over the ...
Structural Heart
This structural heart channel includes news, videos, podcasts and other content related to diagnosis and treatment of structural heart disease. Topics covered include heart valve repair and replacement, transcatheter aortic valve replacement (TAVR), transcatheter mitral valve replacement (TMVR), transcatheter tricuspid valve replacement (TTVR), left atrial appendage (LAA) occlusion, heart failure interventional device therapies, and closing holes in the heart using, including occlusion of atrial septal defects (ASDs), ventricular septal defects (VSDs) and patent foramen ovales (PFOs).

March 17, 2015 — New two-year data from the CoreValve U.S. Pivotal High Risk Study, which demonstrated superior two-year ...

March 17, 2015 — Five-year data suggest that the Sapien transcatheter heart valve (Edwards Lifesciences) is a feasible ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...
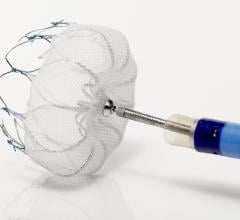
March 16, 2015 — Boston Scientific Corp. has received U.S. Food and Drug Administration (FDA) approval for the Watchman ...
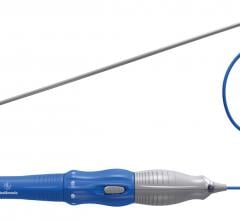
March 13, 2015 — AtriCure Inc. announced the first two patients in the Dual Epicardial and Endocardial Procedure (DEEP) ...

Key data from many transcatheter aortic valve replacement (TAVR) clinical trials will be presented as late-breakers at ...
March 10, 2015 — In an analysis of outcomes of about 12,000 patients who underwent transcatheter aortic valve ...

February 27, 2015 — Four-month-old Jackson Lane has a large scar that looks like a giant zipper down the center of his ...
February 24, 2015 — In light of a recent American Heart Association report stating that U.S. mitral valve regurgitation ...
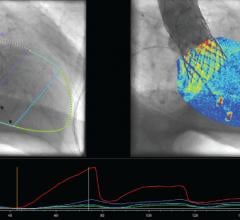
February 23, 2015 — Pie Medical Imaging BV announced that it received 510(k) clearance from the U.S. Food and Drug ...
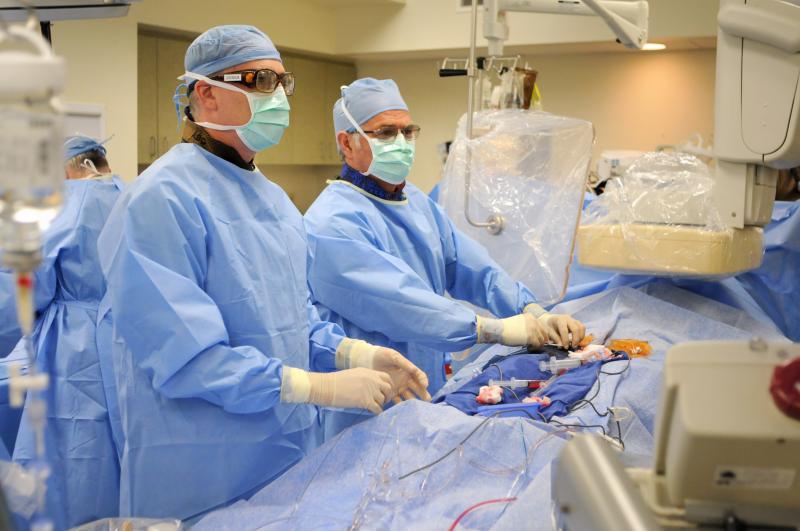
As editor of DAIC, I keep a close watch on trends in cardiovascular technology and try to predict what the next big ...
February 9, 2015 — Medtronic announced CE (Conformité Européene) mark for the 26 and 29 mm sizes of the CoreValve Evolut ...
February 9, 2015 — A minimally invasive procedure to repair severe mitral valve regurgitation by clipping together the ...
January 29, 2015 — Medtronic revealed new one-year clinical data showing that transcatheter aortic valve replacement ...


 March 17, 2015
March 17, 2015