Structural Heart
This structural heart channel includes news, videos, podcasts and other content related to diagnosis and treatment of structural heart disease. Topics covered include heart valve repair and replacement, transcatheter aortic valve replacement (TAVR), transcatheter mitral valve replacement (TMVR), transcatheter tricuspid valve replacement (TTVR), left atrial appendage (LAA) occlusion, heart failure interventional device therapies, and closing holes in the heart using, including occlusion of atrial septal defects (ASDs), ventricular septal defects (VSDs) and patent foramen ovales (PFOs).
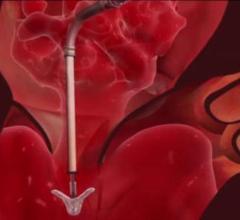
This animation, provided by Abbott Vascular, demonstrates how the U.S. Food and Drug Administration (FDA)-cleared ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...

October 22, 2013 — Medtronic Inc. announced the first implants in the CoreValve Evolut R clinical study, which will ...

October 11, 2013 — Bioventrix announced the presentation of scientific data demonstrating the durability of its Revivent ...

October 10, 2013 — A mother and her 25-week-old fetus are doing well after a team of physicians performed a successful i ...

Oct. 1, 2013 – The Cardiovascular Research Foundation (CRF) announced the late breaking trials and first report ...
September 30, 2013 --CardioLogical Solutions announced that it has been issued two additional patents for its family of ...
September 27, 2013 – JenaValve Technology, Inc. announced that it has raised $62.5 million in a Series C venture round.
T ...
September 24, 2013 – Edwards Lifesciences Corp. announced a new post-hoc data analysis from The PARTNER Trial ...


 October 28, 2013
October 28, 2013