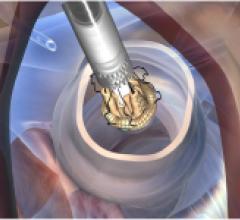
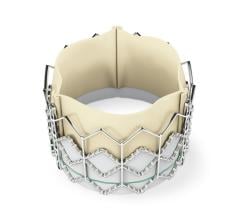
May 28, 2013 — CardioKinetix Inc. has announced the results of a meta-analysis study of the catheter-based Parachute ...
Structural Heart
This structural heart channel includes news, videos, podcasts and other content related to diagnosis and treatment of structural heart disease. Topics covered include heart valve repair and replacement, transcatheter aortic valve replacement (TAVR), transcatheter mitral valve replacement (TMVR), transcatheter tricuspid valve replacement (TTVR), left atrial appendage (LAA) occlusion, heart failure interventional device therapies, and closing holes in the heart using, including occlusion of atrial septal defects (ASDs), ventricular septal defects (VSDs) and patent foramen ovales (PFOs).
May 24, 2013 — Neovasc Inc. reported positive animal data from its Tiara program for the transcatheter treatment of ...
May 16, 2013 — Sorin Group has received U.S. Food and Drug Administration (FDA) approval of its Investigational Device ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...
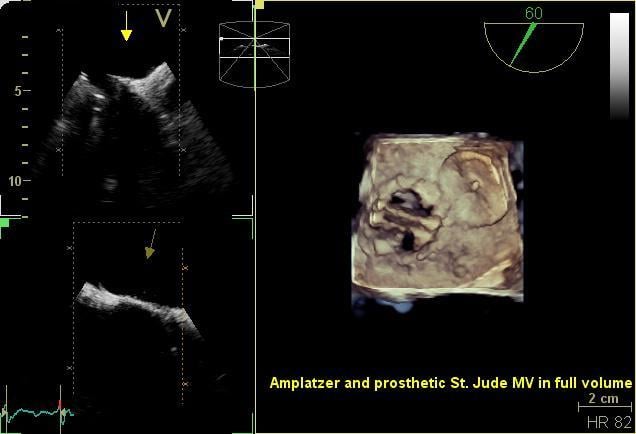
The development of 3-D transesophageal echo (TEE) just a few years ago has enabled a new generation of interventional ...
May 13, 2013 — Live demonstration, transmission or recording of transcatheter aortic valve replacement (TAVR) has been ...

May 13, 2013 — Boston Scientific reports that the four-year follow-up data from the PROTECT AF clinical trial demonstrat ...
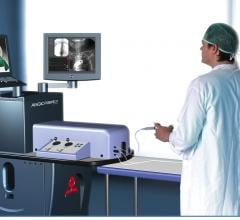
May 2, 2013 — Simbionix USA Corp. released various training modules for the Angio Mentor endovascular simulator.
The ...
May 1, 2013 — InterValve Inc. announced that it has received 510(k) clearance to market the new V8 Aortic Valvuloplasty ...
April 17, 2013 — Multi-detector computed tomography (MDCT) is a better way to measure annular size in patients with ...
April 12, 2013 — Colibri Heart Valve LLC has successfully completed the first clinical use of the company's proprietary ...
April 12, 2013 — NeoChord has received CE mark for its technology that allows the implantation of artificial chordae ...
April 3, 2013 — An RTI International-developed prototype catheter that can generate live, streaming 3-D ultrasound ...
March 28, 2013 — St. Jude Medical announced publication of results from its landmark RESPECT clinical trial in The New ...
March 27, 2013 — The Heart Failure 2013 LA meeting will include a showcase of several startup companies offering ...
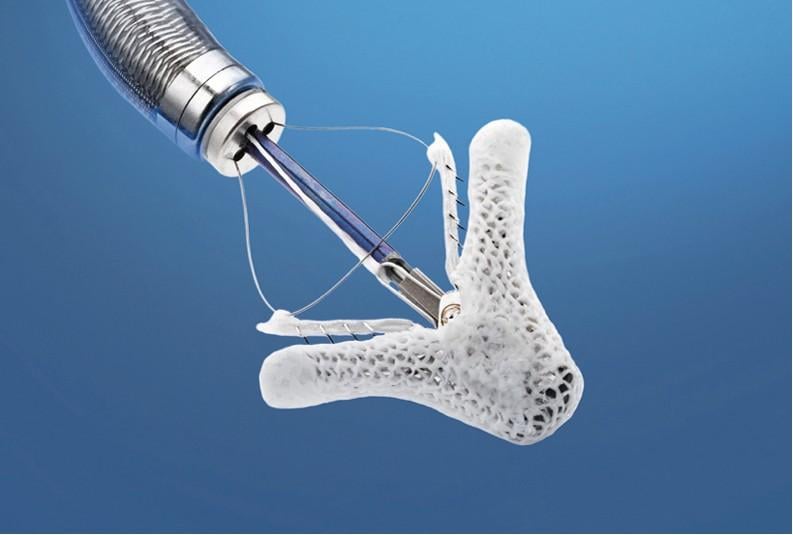
March 22, 2013 — Abbott announced that the U.S. Food and Drug Administration (FDA) Circulatory System Devices Panel of ...


 May 28, 2013
May 28, 2013