April 3, 2013 — An RTI International-developed prototype catheter that can generate live, streaming 3-D ultrasound images from inside the heart received a Cardiovascular Innovation Award at the 2013 Cardiovascular Research Technologies Annual Symposium.
The technology, called a live volumetric imaging (LVI) intracardiac catheter has the potential to improve catheter-based interventional heart procedures such as transcatheter valve therapies and cardiac ablation for arrhythmias. LVI was one of three technologies recognized at the symposium.
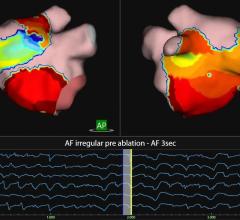
Existing intracardiac echo catheters produce two-dimensional image “slices” that limit a surgeon’s field of view. Using innovative matrix transducer array technology, LVI offers a much wider field of view with a high frame rate and deep penetration depth, which allows the surgeon to see many angles of the heart.
“The goal is to enable better image guidance to improve accuracy and reduce complications for procedures that are done today with limited direct visualization of the tissue,” said David Dausch, Ph.D., a senior research engineer at RTI International and lead inventor of the technology.
The novel matrix transducer array, called a piezoelectric micromachined ultrasound transducer, is made using semiconductor fabrication techniques. Located at the tip of a catheter inserted into the heart, the transducer generates the acoustic signals that are processed by an ultrasound system to produce the live 3-D image.
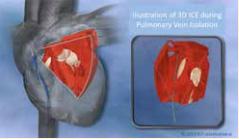
When inserted through the femoral vein and advanced into the right atrium, the catheter provides a real-time volume view of the left atrium and ventricle. The image can then be rotated to show any arbitrary angle within this volume view without repositioning the catheter.
“We believe LVI can enable imaging of full cardiac volumes from within the heart, with high frame rate to capture cardiac dynamics, and resolution sufficient to clearly visualize endocardial structures and track therapeutic tools during interventional procedures,” Dausch said.
RTI is currently seeking partners interested in commercializing the LVI platform for medical applications.
For more information: www.rti.org/lvi


 September 05, 2025
September 05, 2025