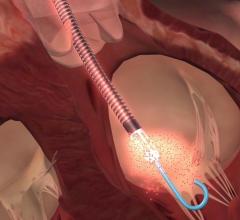
March 10, 2020 — The U.S. Food and Drug Administration (FDA) has cleared the SynCardia Systems 50cc temporary Total ...
Ventricular Assist Devices (VAD)
This channel includes news and new technology innovations for ventricular assist devices (VAD). VADs are a type of mechanical hemodynamic support device that helps increase blood flow in people who have ventricles that are not work properly due to heart failure, cardiogenic shock, cardiomyopathy or myocardial infarction. Most often these devices support the left ventricle, so they are often referred to as left ventricular assist devices (LVAD). VADS come in two types, surgically implanted, usually as a bridge to heart transplant, and percutanenous catheter-based pumps used for temporary hemodynamic support. Examples of temporary percutaneous pumps include the Impella and TandemHeart devices.
March 5, 2020 — Abbott recently received Breakthrough Device designation from the U.S. Food and Drug Administration (FDA ...
February 12, 2020 — The U.S. Food and Drug Administration (FDA) has approved Carmat's investigational device exemption ...

DAIC Editor Dave Fornell recently took a tour of the Abiomed Impella production line at its headquarters in Danvers ...
Interview with Navin Kapur, M.D., FAHA, FACC, FSCAI, executive director, The CardioVascular Center for Research and ...
Navin Kapur, M.D., FAHA, FACC, FSCAI, director, Acute Mechanical Circulatory Support Program and executive director of ...
Haval Chweich, M.D., medical director of the cardiac critical care unit (CCU) at Tufts Medical Center, and assistant ...
Richard Botto, CVT, RCSA, chief cardiovascular technologist, division of cardiology, cardiac cath lab, and Melissa Smith ...
January 7, 2020 — Abbott announced U.S. Food and Drug Administration (FDA) approval of a new alternative surgical ...
November 4, 2019 — Abbott is recalling its CentriMag Acute Circulatory Support System due to a calibration system error ...
October 28, 2019 — Nearly two years of real-world outcomes data on Abiomed’s Impella RP heart pump shows that when ...
Jeffrey J. Popma, M.D., director of interventional cardiology clinical services at Beth Israel Deaconess Medical Center ...
William O’Neill, M.D., medical director of the Center for Structural Heart Disease at Henry Ford Hospital, Detroit ...
September 27, 2019 — Abiomed’s newest heart pump, the Impella 5.5 with SmartAssist, has received U.S. Food and Drug ...
September 12, 2019 — CorWave announced successful completion of its first 60-day preclinical study to evaluate its ...

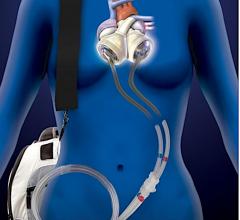
 March 10, 2020
March 10, 2020