August 5, 2022 — Black people and women with severe heart failure who might be good candidates for surgery to implant a ...
Ventricular Assist Devices (VAD)
This channel includes news and new technology innovations for ventricular assist devices (VAD). VADs are a type of mechanical hemodynamic support device that helps increase blood flow in people who have ventricles that are not work properly due to heart failure, cardiogenic shock, cardiomyopathy or myocardial infarction. Most often these devices support the left ventricle, so they are often referred to as left ventricular assist devices (LVAD). VADS come in two types, surgically implanted, usually as a bridge to heart transplant, and percutanenous catheter-based pumps used for temporary hemodynamic support. Examples of temporary percutaneous pumps include the Impella and TandemHeart devices.
July 14, 2022 — University of Toronto Engineering researchers have grown a small-scale model of a human left heart ...
October 18, 2022 — The U.S. Food and Drug Administration (FDA) is providing updates to the FDA website to include ...
June 23, 2022 — Medtronic, Inc. is recalling a single lot of HeartWare HVAD System batteries due to welding defects that ...
June 8, 2022 — The U.S. Food and Drug Administration (FDA) has issued a release stating that Medtronic is recalling the ...
May 25, 2022 — ŌNŌCOR LLC, a leader in endovascular safety technology, today announced it has received 510(k) U.S. Food ...
April 28, 2022 — The U.S. Food and Drug Administration (FDA) is alerting healthcare providers to the possibility that ...
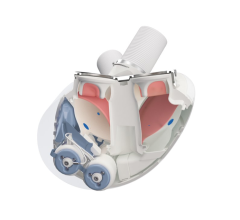
March 28, 2022 — Carmat, the designer and developer of the world’s most advanced total artificial heart, aiming to ...
Srihari Naidu, M.D., FACC, FAHA, FSCAI, is the director of both the cardiac cath labs and Hypertrophic Cardiomyopathy ...
October 20, 2021 – CorWave, a French medtech company developing a next-generation heart pump, won the 2021 HealthTech ...
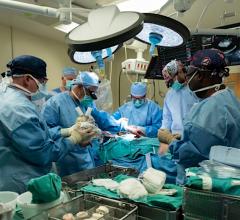
September 22, 2021 — A cardiothoracic surgical team with University of Louisville Health – Jewish Hospital has performed ...

Medtronic announced in June it was stopping the sale and distribution of the Medtronic Heartware HVAD left ventricular ...
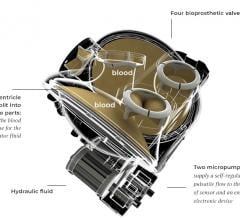
June 18, 2021 – An experimental artificial heart includes an autoregulation control mechanism, or Auto-Mode, that can ...
June 21, 2021 — The U.S. Food and Drug Administration (FDA) approved Boehringer Ingelheim's dabigatran etexilate ...
Surgeons at Penn State Heart and Vascular Institute were the second group in the nation to implant a newly-designed ...


 August 05, 2022
August 05, 2022