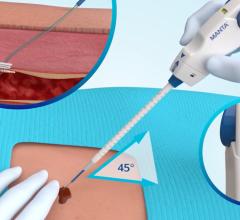
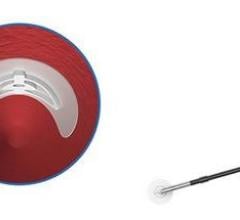
June 6, 2011 - AccessClosure Inc. announced an exclusive agreement with Biosensors International for the distribution of the Mynx Vascular Closure Device throughout the United Kingdom, Switzerland and France. The extravascular Mynx device, designed for patient comfort while providing hemostasis without sutures or implants, will be sold as part of Biosensors' interventional cardiology product range.
Biosensors will join AccessClosure's existing global network of distributors. Biosensors is now the fifth largest drug-eluting stent (DES) supplier globally, marketing its BioMatrix family of DES systems which offer the unique combination of Biolimus A9, an anti-restenotic drug developed by Biosensors exclusively for use with DES, and an abluminally-coated biodegradable polymer. Biosensors has a well established direct presence in the UK, Switzerland and France.
The Mynx Vascular Closure Device utilizes a conformable, water-soluble polyethylene glycol (PEG) sealant to immediately seal the femoral artery. Dissolving within 30 days, Mynx leaves nothing behind but a healed artery. The extravascular device is designed for increased patient comfort and clinical versatility. The Mynx, which received its first U.S Food and Drug Administration (FDA) approval in May 2007, has been used in over 800,000 procedures to date.
For more information: www.accessclosure.com

 October 07, 2025
October 07, 2025