March 9, 2017 — Metro Detroit cardiologists from five health systems have joined together to increase residents’ survival rate from heart attacks.
The Detroit Cardiogenic Shock Initiative doubled survival rates in patients experiencing a life-threatening side-effect to a heart attack, the group announced recently in Detroit.
“This unprecedented effort shows the powerful advances we can make to save lives by working together in metro Detroit,” said lead investigator William W. O’Neill, M.D., director of the Center for Structural Heart Disease at Henry Ford Health System.
The initiative addresses cardiogenic shock, a devastating complication of heart attacks. In these patients, the pump function of the heart is severely depressed, causing low blood pressure and vital organs to be deprived of sufficient blood supply. Despite contemporary treatments, about 50 percent of patients experiencing the condition die.
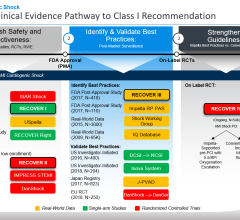
The health systems participating in the initiative have agreed to use a heart pump the size of a straw to keep blood pumping throughout the body. The Impella pump, a U.S. Food and Drug Administration (FDA)-approved device, is inserted through a catheter in the groin into the heart as soon as the patient arrives at the hospital. Doctors then treat the cause of the heart attack, either inserting a stent, removing a clot or taking other necessary action while the tiny pump supports circulation in the rest of the body.
The key difference with this initiative is that the Impella system is placed before standard treatment to fix the blocked artery.
“There is no question in our minds that early circulatory support is critical to improve the chance of a successful outcome in these critically ill patients,” said Simon Dixon, M.D., chair of cardiovascular medicine at Beaumont Hospital, Royal Oak, Mich.
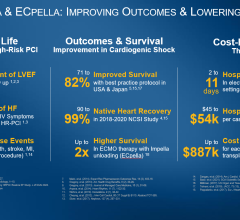
Since the initiative began in July 2016, doctors at the participating hospitals found the approach was the most appropriate way to treat 30 patients who were having a heart attack and showing signs of cardiogenic shock. The treatment resulted in an 80 percent survival rate, compared to the traditional 50 percent survival rate.
“Cardiologists have been trying to move the needle on heart attack survival rate for years; this is a huge success,” said Thomas Lalonde, M.D., chief of cardiology at St. John Hospital in Detroit.
Superior Air-Ground Ambulance Service, Inc. is also supporting the initiative when transporting heart attack patients showing signs of cardiogenic shock.
O’Neill is scheduled to mention the initiative’s results in a presentation at the annual American College of Cardiology (ACC) Scientific Sessions, March 17-19 in Washington, D.C. Cardiologists in Boston, Los Angeles and other cities in the United States are following the initiative closely, to replicate for patients there, O’Neill said.
"This is a chance for a number of excellent programs from southeast Michigan to collaborate for the benefit of our patients," said Arthur Szyniszewski, M.D., cardiologist at Saint Joseph Mercy Health System.
Watch a VIDEO demonstration of how the Impella works.
The read article "Study Finds Impella Heart Pump Reduces Kidney Injury Risk During High-Risk PCI."
For more information: www.henryford.com


 April 28, 2023
April 28, 2023