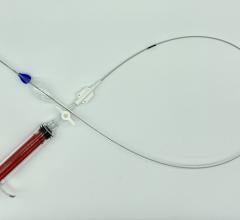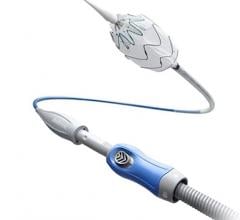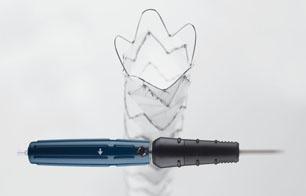
Zenith Alpha Thoracic Endovascular Graft image courtesy of Cook Medical
September 18, 2015 —Cook Medical has received premarket approval from the U.S. Food and Drug Administration (FDA) for its lower-profile Zenith Alpha Thoracic endovascular graft. Zenith Alpha Thoracic is indicated for the endovascular treatment of patients with isolated lesions of the descending thoracic aorta (not including dissections) having vascular anatomy suitable for endovascular repair. The approval of Zenith Alpha Thoracic was based on two pivotal clinical trials that studied the safety and effectiveness of the device in patients with aortic aneurysm/ulcer or blunt traumatic aortic injury.
The device will allow physicians to treat more patients with thoracic endovascular aortic repair (TEVAR) because of its lower-profile introduction system and broad range of sizes. With a 16-20 French delivery system, Zenith Alpha Thoracic was developed to address vascular access issues associated with larger-profile devices and to increase conformability in tortuous anatomy.
The device’s introduction system also features an ergonomic design that requires fewer procedural steps than previous designs to deploy the device without sacrificing the precision and control of the Zenith platform.
“Despite all the successes we’ve seen over the past few decades in endovascular aneurysm repair, we continue to be frustrated when we have to put a large sheath through iliac arteries that are smaller than the delivery system,” said Karl Illig, M.D., professor of surgery and director of the division of vascular surgery at USF Morsani College of Medicine. “Development, testing and now approval of the Zenith Alpha Thoracic device is long-awaited in this regard.”
Zenith Alpha Thoracic was first launched in Europe following CE Mark approval in 2013, and is the newest addition to a growing portfolio of clinically proven Zenith endovascular grafts and procedural accessories in the United States.
Illig is a paid global principal investigator of the Zenith Alpha Thoracic clinical trial.
For more information: www.cookmedical.com


 September 18, 2025
September 18, 2025