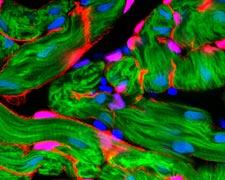
October 13, 2009 – By mimicking the way embryonic stem cells develop into heart muscle in a lab, Duke University bioengineers believe they have taken an important first step toward growing a living heart patch to repair heart tissue damaged by disease.
In a series of experiments using mouse embryonic stem cells, the bioengineers used a novel mold of their own design to fashion a three-dimensional patch made up of heart muscle cells, known as cardiomyocytes. The new tissue exhibited the two most important attributes of heart muscle cells – the ability to contract and to conduct electrical impulses. The mold looks much like a piece of Chex cereal in which researchers varied the shape and length of the pores to control the direction and orientation of the growing cells.
The researchers grew the cells in an environment much like that found in natural tissues. They encapsulated the cells within a gel composed of the blood-clotting protein fibrin, which provided mechanical support to the cells, allowing them to form a three-dimensional structure. They also found that the cardiomyocytes flourished only in the presence of a class of helper cells known as cardiac fibroblasts, which comprise as much as 60 percent of all cells present in a human heart.
“If you tried to grow cardiomyocytes alone, they develop into an unorganized ball of cells,” said Brian Liau, graduate student in biomedical engineering at Duke’s Pratt School of Engineering. Liau, who works in the laboratory of assistant professor Nenad Bursac, presented the results of his latest experiments during the annual scientific sessions of the Biomedical Engineering Society in Pittsburgh.
“We found that adding cardiac fibroblasts to the growing cardiomyocytes created a nourishing environment that stimulated the cells to grow as if they were in a developing heart,” Liau said. “When we tested the patch, we found that because the cells aligned themselves in the same direction, they were able to contract like native cells. They were also able to carry the electrical signals that make cardiomyocytes function in a coordinated fashion.”
“The addition of fibroblasts in our experiments provided signals that we believe are present in a developing embryo,” Liau said. The need for helper cells is not uncommon in mammalian development. For example, he explained, nerve cells need “sheathe” cells known as glia in order to develop and function properly.
Bursac believes that the latest experiments represent a proof-of-principle advance, but said there are still many hurdles to overcome before such patches could be implanted into humans with heart disease.
“While we were able to grow heart muscle cells that were able to contract with strength and carry electric impulses quickly, there are many other factors that need to be considered,” Bursac said. “The use of fibrin as a structural material allowed us to grow thicker, three-dimensional patches, which would be essential for the delivery of therapeutic doses of cells. One of the major challenges then would be establishing a blood vessel supply to sustain the patch.”
The researchers plan to test their model using nonembryonic stem cells. For use in humans, this is important for many reasons, both scientifically and ethically, Bursac said. Recent studies have demonstrated that some cells from human adults have the ability to be reprogrammed to become similar to embryonic stem cells.
“Human cardiomyocytes tend to grow a lot slower than those of mice,” Bursac said. “Since it takes nine months for the human heart to complete development, we need to find a way to get the cells to grow faster while maintaining the same essential properties of native cells.”
If they could use a patient’s own cells, the patch would also evade an immune system reaction, Bursac added.
The research was supported by National Institutes of Health, the National Heart Lung Blood Institute and Duke’s Stem Cell Innovation program. Other Duke members of the research team were Weining Bian and Nicolas Christoforou.
For more information: www.stemcell.duke.edu


 November 19, 2021
November 19, 2021 









