September 11, 2014 — Geneia announced a multi-year, strategic partnership with Covidien to help improve the health of and reduce the costs for chronically ill patients. Covidien has agreed to provide Geneia with rights to sell its ZephyrLIFE remote patient monitoring solutions in designated markets to monitor patients with heart disease, diabetes and other chronic illnesses in the home setting.
"At Geneia, we're creating a family of population health solutions that identify who needs help, exactly when they need it, and what they need to improve their health. We're thrilled to partner with Covidien, a global leader in patient monitoring, to power @home, Geneia's next generation remote patient monitoring solution for the $20.9 billion remote patient monitoring market [1]," said Heather Lavoie, president and chief operating officer of Geneia. "As a result of this partnership, we're marrying Geneia's clinical care expertise with Covidien's innovative ZephyrLIFE technology to help improve the quality and cost of care for chronically ill patients in the home setting."
David J. Giarracco, Covidien's vice president of market development for patient monitoring, agreed. "The Covidien – Geneia partnership is a powerful combination of health care expertise with a singular focus on the patient. We are excited to extend our technology from the acute care, hospital environment into the home setting. Together with Geneia, we're deploying a solution that is aimed to help improve health outcomes and reduce cost of caring for patients with congestive heart failure, diabetes, and other chronic diseases, all while the patient is at home."
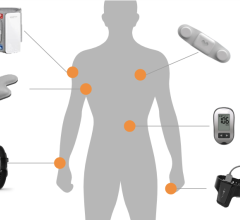
Geneia's @home product combines Covidien's ZephyrLIFE remote monitoring technology with Geneia's clinical care expertise to better manage chronic and acute conditions. Patients use the @home remote monitoring device to record their vital signs on a daily basis, including weight, pulse, blood pressure, blood oxygen level and blood glucose. Geneia merges this patient data with administrative and clinical data sets to provide an early warning about patients at risk and trigger the deployment of early intervention strategies such as telephonic support and home visits by clinicians. As a result, @home is able to:
- Reduce hospital admissions/readmissions, observation bed days and emergency department visits;
- Ensure appropriate medication usage;
- Improve adherence to recommended preventive care;
- Improve coordination of care between the patient and their physician(s); and
- Enable and engage patients and the people in their support system to participate in managing their health and well-being.
Geneia also announced that Capital BlueCross, a $3 billion health plan with more than 700,000 members, is the first of the Blue plans to pilot @home to monitor patients with congestive heart failure. "At Capital BlueCross, our members afflicted with congestive heart failure are among the sickest and costliest members we have. We're confident that @home will improve the health outcomes and reduce hospital readmissions for these members," said Dr. Jennifer Chambers, senior vice president and chief medical officer of Capital BlueCross.
This service is now available to other Blue plans and insurance carriers across the country as a value-add service to chronically ill patients.
For more information: geneia.com
References:
[1] Lewis, Nicole, "Remote Patient Monitoring Market to Double by 2016." InformationWeek. 24 July 2012.


 September 16, 2025
September 16, 2025