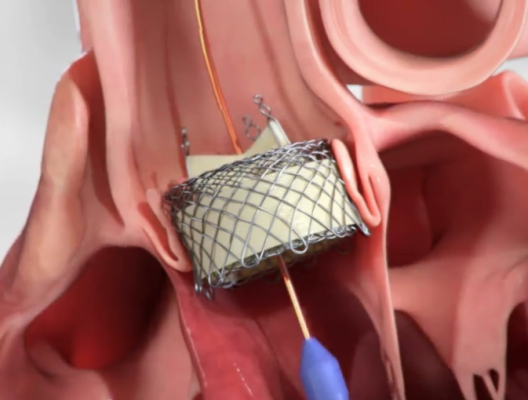
March 24, 2022 — HLT Inc., a Bracco Group Company and a leader in the development of cutting edge transcatheter aortic valve replacement (TAVR) therapy, announced today that it has received U.S. Food and Drug Administration (FDA) approval for two clinical studies to assess the performance and safety of its HLT Meridian TAVR Valve System to treat aortic stenosis and aortic regurgitation among high-risk patients suffering from aortic disease.
“We are thrilled to be providing a minimally invasive option to patients suffering from aortic disease,” said David R. Elizondo, President and CEO of HLT. “Currently, there are treatments available that focus specifically on aortic stenosis. The HLT technology has been designed to treat a much broader segment in aortic disease where currently treatment options are limited. The HLT Meridian TAVR Valve System is designed to address both patients’ and physicians’ needs that are unmet by current offerings, and is intended to expand and optimize the clinical and procedural performance.”
Aortic stenosis, a narrowing of the aortic valve that obstructs blood flow from the heart, is one of the most common heart valve diseases. Approximately 250,000 people in the U.S. are diagnosed annually with symptomatic severe aortic stenosis (SSAS). Aortic regurgitation is when an aortic valve does not close properly, resulting in reverse blood flow from the aorta back into the left ventricle. Surgical aortic valve replacement (SAVR) is the current standard treatment for these patients. However, TAVR is a minimally invasive way to replace the diseased aortic valve without open heart surgery for this patient population.
Dr. Satya Shreenivas, Chief Medical Officer at HLT and experienced interventional cardiologist with an expertise in the care of patients with structural heart disease, added, “The HLT platform is exciting because its new single operator delivery system and unique valve design offer the promise of a more accurate, and thus possibly less complicated procedure, and better long-term outcomes due to the intended reduced stress on the individual valve leaflets. In addition, the delivery system is designed to allow precision in valve delivery. This, combined with the radial strength of the valve, are expected to be a great combination to treat non-calcified aortic annuli that are frequently seen in aortic regurgitation.”
Current TAVR options require multiple operators for deployment, do not allow for complete repositioning or assessment prior to implant and cannot treat expanded indications, such as aortic regurgitation. Furthermore, the patients are left with concerning rates of adverse events such as the need for permanent pacemaker implantation, paravalvular leakage, and patient-prosthesis mismatch, all of which cause concern. Unlike current TAVR solutions, the HLT Meridian TAVR Valve System is designed to have the benefits of both balloon expandable and self-expanding platforms with a lower profile and non-obstructive design that reduces coronary and conduction obstruction. Its design for increased hemodynamic performance is expected to reduce the concern of patient-prosthesis mismatch. It has a unique design as the only TAVR valve with an isolated valve structure where the leaflets are not attached to an annular structure. The novel design is intended to reduce tissue stress, improve long-term durability, minimize leakage and minimize ventricular exposure.
As stated above, the HLT Meridian TAVR Valve System is not yet cleared/ approved by FDA. It is pending clinical investigation and will be reviewed for clearance/approval in the future.
For more information: www.hltmedical.com


 July 08, 2024
July 08, 2024 









