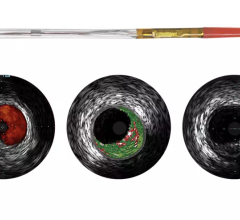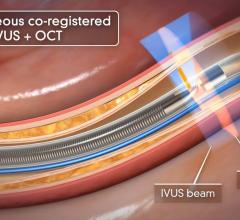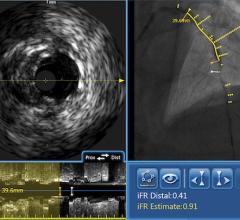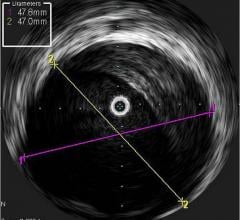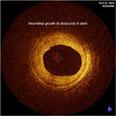
An example of an intravascular OCT image using the LightLab system.
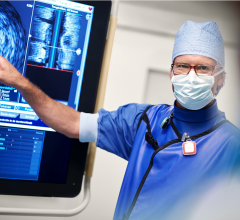
May 21, 2009 - LightLab Imaging Inc. this week is launching its next generation optical coherence tomography (OCT) system, the C7XR FD-OCT Imaging System with the C7 Dragonfly Imaging Catheter, at EuroPCR 2009 in Barcelona, Spain.
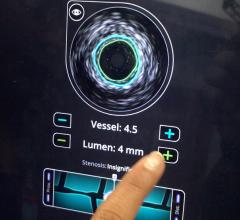
The C7XR FD-OCT system is the world’s first approved frequency domain OCT product for intracoronary imaging, having received CE mark in March. The C7XR FD-OCT and C7 Dragonfly imaging catheter enables micron level resolution imaging of the coronary artery with the capability to scan a 40 mm artery segment in less than three seconds with a single nonocclusive catheter.
“The C7XR system and C7 Dragonfly imaging catheter represent a significant step forward for intracoronary imaging,” said Dr. Francesco Prati, Ospedale San Giovanni, Rome. “I am very pleased with the speed and simplicity of the imaging procedure and the improvements in image quality are truly impressive. I would expect frequency domain OCT to replace IVUS in many cases or centers.”
OCT generates intravascular images with a resolution up to 10 to 15 times greater than other clinically available imaging devices, including intravascular ultrasound (IVUS). With its higher resolution, OCT may reduce observer variability in all standard cross-sectional measurements.
The C7XR system follows the M2x OCT Imaging System, LightLab’s Time Domain OCT (TD-OCT) device, which is available in more than 20 countries worldwide. More than 10,000 patient intracoronary images have been taken since the company launched the M2x. Until the launch of the C7XR FD-OCT Imaging System the M2x was the only commercially available intracoronary OCT imaging device in the world. The C7XR system is 10 times faster and has a higher resolution. Unlike previous OCT systems, the new release can image vessels without the need for vessel occlusion, which was a big complaint and drawback of previous OCT systems.
For more information: www.lightlabimaging.com


 September 18, 2025
September 18, 2025