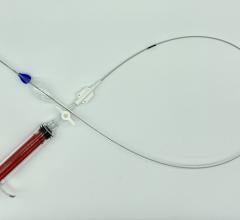
December 13, 2016 — Lombard Medical Inc. announced that its Altura endovascular stent graft system was featured in a scientific presentation at the 43rd annual VEITHsymposium, Nov. 15-19 in New York City. The Altura stent graft, which was launched commercially in Europe earlier in 2016, is specifically designed to simplify treatment in patients with normal abdominal aortic aneurysm (AAA) anatomy.
The Altura stent graft was featured in a scientific presentation by David Murray, consultant vascular surgeon at Manchester Royal Infirmary, Manchester, U.K., titled, “Advantages and Limitations of Lombard’s Altura Endograft Device to Simplify EVAR Procedures: A Multicenter Study.”
The data showed a total of 24 patients were treated electively between April and October 2016. Sixteen patients were male, with a mean age of 75. Median AAA diameter was 60mm (52-105mm), median neck length was 23mm (15-60mm), median proximal neck diameter was 23mm (19-26mm) and median β neck angulation was 31 degrees. Median iliac landing zone diameter was 14mm (9-18mm). Access was percutaneous in 25 of 38 groins.
Results indicated:
- Eighteen (75 percent) of patients were discharged at or before 24 hours (Day-Case EVAR [endovascular aneurysm repair);
- There were no deaths within 90 days; and
- There were no immediate or delayed access-related complications.
Screening times and radiation doses were low and showed a reduction over time and experience with the device:
- Deployment time ranged 19-45 minutes;
- Mean contrast dose was 150ml;
- Median intraoperative contrast dose was 75ml of 50 percent concentrate Visipaque 270; and
- A further 60ml for rotational angiography at completion in the hybrid lab.
Technical success was 100 percent with freedom from type I/III endoleaks at 100 percent with stable aneurysm size and no evidence of stent-graft migration.
Murray commented, “The significance of the low contrast doses, low screen and procedure times, and radiation dose reduction already demonstrates some of the key benefits the new Altura system offers, even taking into account that this data includes the learning curve across two units. It has the potential to be a game-changer for EVAR as we have already been able to perform two-thirds of our Altura cases as ‘day-case’ procedures.”
Steve Richardson, consultant vascular surgeon at University Hospital South Manchester (UHSM), U.K., added, “We are extremely pleased with our early experience using Altura. The learning curve is rapid and it has quickly become the go-to endograft in our vascular unit.”
Delivered via an ultra-low profile 14F catheter, Altura allows for repositioning during deployment and accurate graft placement at each renal artery, enabling physicians to utilize all of the available aortic neck. It also eliminates the need for cannulation that results in a simple, safe and consistent deployment with predictable, shorter procedure times. With just six product sizes, the Altura system allows the majority of patients who present for EVAR repair to be treated quickly with minimal hospital stay and recovery times.
The Altura system received CE Mark in 2015. Lombard launched the device in the United Kingdom and Germany in February 2016 with a broader international rollout currently underway.
For more information: www.lombardmedical.com


 September 18, 2025
September 18, 2025