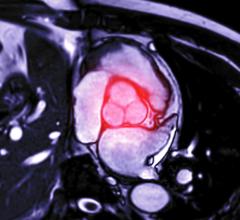
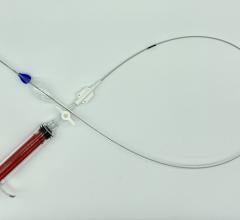
Heli-FX image courtesy of Aptus Endosystems
July 2, 2015 - Medtronic plc announced it has acquired the assets of Aptus Endosystems Inc., a privately held medical device company focused on developing advanced technology for endovascular aneurysm repair (EVAR) and thoracic endovascular aneurysm repair (TEVAR). Medtronic completed its acquisition of the assets of Aptus Endosystems in a transaction valued at approximately $110 million. Additional terms of the acquisition were not disclosed.
Aptus Endosystems' Heli-FX and Heli-FX Thoracic EndoAnchor systems feature an endovascular-deployed anchor designed to attach a variety of aortic endografts to the native vessel wall. This off-the-shelf, customized solution minimizes the need for complicated procedures for the select subset of patients who would benefit from supplementary fixation. This may include patients with challenging anatomies, risk factors for a secondary intervention and existing seal complications, as well as in situations where a physician may intraoperatively determine the need for additional security.
The Heli-FX and Heli-FX Thoracic EndoAnchor systems bear the CE Mark for distribution in the European Union and are cleared by the U.S. Food and Drug Administration (FDA) for distribution in the United States. Both devices can be used with a wide variety of commercially available stent grafts, including Medtronic's Endurant and Valiant stent graft systems.
As part of the acquisition, Medtronic will also distribute the TourGuide Steerable Sheath, a device with adjustable tip that enables quick access and delivery of peripheral vascular products to the most challenging anatomy, in the United States and Europe.
Medtronic will continue the ANCHOR Registry - a global, multi-center, prospective post-market registry evaluating the Heli-FX Aortic Securement System. The company also plans to pursue approval for <10mm infrarenal proximal neck indication for Endurant with the use of the Heli-FX EndoAnchor system.
Medtronic will report the Aptus business as part of its Aortic and Peripheral Vascular division within the Cardiac and Vascular Group. The transaction is expected to meet Medtronic's long-term financial metrics for acquisitions, and the annualized earnings impact of this acquisition is not expected to be material.
For more information: www.medtronic.com


 September 18, 2025
September 18, 2025