October 24, 2011 — NeoStem Inc. and Amorcyte Inc announced the closing of their previously publicized merger transaction following approval by the shareholders of both companies on Oct. 14, 2011. NeoStem closed on a financing raising $16.5 million in gross proceeds on July 22. The company intends to initiate, no later than the first quarter of 2012, a Phase 2 clinical trial for Amorcyte's lead product candidate, AMR-001, for the treatment of acute myocardial infarction (AMI).
Pursuant to the terms of the Merger Agreement, NeoStem (i) issued 5,843,483 shares as the base stock consideration; (ii) will issue up to 4,092,768 shares of common stock upon the achievement of certain specified business milestones; (iii) issued warrants to purchase 1,881,008 shares of common stock exercisable over a seven-year period at a price of $1.466 per share; and (iv) upon the successful commercialization of AMR-001 will pay certain earn out payments on sales. The shares of common stock issued and underlying the warrants are subject to certain transfer restrictions.
Of the approximately 800,000 Americans who suffer an AMI each year, approximately 20 percent, or 160,000 patients, remain at risk for progressive deterioration in heart muscle function; as a consequence, they are also at increased risk for future major adverse cardiac events. AMR-001 targets treatment of this unmet medical need.
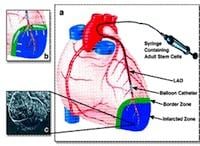
AMR-001 is an autologous, bone marrow-derived, pharmaceutical grade cell-based product that uses a cell population enriched for CD34+CXCR4+ cells. Studies have shown that these cells act as a natural repair mechanism, releasing from bone marrow and traveling to the damaged region of the heart following an AMI.
Treatment with AMR-001 involves infusion of an active population of these cells directly into a patient's heart via an intra-coronary catheter six to eleven days after an AMI (i.e., after the "hot" or inflammatory phase). This complements the body's natural rescue mechanism for those cells that face hypoxic stress (i.e., oxygen deprivation) as a result of an increased workload.
NeoStem believes AMR-001 stands alone in its ability to claim all of the following attributes:
- A confirmed mechanism of action
- Access to a cGMP (current good manufacturing practices) facility
- An established dose exceeding the threshold for biological activity
- Use of autologous cells with no risk of rejection and capable of integrating and providing local support, potentially for a prolonged period of time as demonstrated in pre-clinical animal experiments
- Cells that are not expanded, thereby eliminating the potential concerns associated with such expansion, and
- An issued patent with composition of matter, methods and processes claims with long remaining life.
For more information: www.neostem.com; www.amorcyte.com


 November 19, 2021
November 19, 2021 









