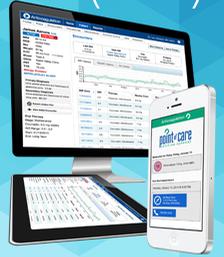
Image courtesy of Point of Care Decision Support
March 11, 2015 — Point of Care Anticoagulation software (PCDS AC) optimizes delivery of anticoagulation (AC) drugs and reduces adverse events associated with anticoagulation therapy. The software was designed and developed in collaboration with leading clinical thrombosis experts, and is a decision support tool that takes the complexity out of AC management by providing a real-time AC dashboard. With intuitive, predictive clinical decision support, PCDS AC transforms evidence-based guidelines into intelligent tools that measure and improve AC therapy outcomes.
PCDS AC meets the key recommendations of The Joint Commission, the Centers for Medicare and Medicaid Services (CMS) and the Anticoagulation EHR (Electronic Health Record) Task Force. The system could be beneficial in light of new target-specfic oral anticoagulants (TSOAC) drugs coming onto the market, changing AC therapy protocols and more stringent regulatory guidelines.
Key benefits and outcomes of using PCDS AC include:
Patient Safety and Cost Savings: Warfarin-related adverse drug events (myocardial infarctions, strokes, major bleeds, etc.) are reduced by consistently achieving time-in-therapeutic range (TTR) above 70 percent per CMS recommendations.
Patient Safety & Revenue Generation: The PCDS AC system brings together the ACCP Antithrombotic guidelines, risk ratings like CHA2DS2-VASc, HAS-BLED, HEMORR2HAGES, drug-drug interactions, and other evidence-based research to automatically identify high risk patients and manage their AC care end-to-end. This also provides an opportunity to identify new patients who may benefit from proactive AC therapy and reduce their overall risk of stroke.
Patient Satisfaction: The PCDS AC system incorporates full TSOAC therapy and workflow, which allow for expanded paradigms of anticoagulant management. These new TSOAC agents require far less blood testing, which is a major benefit for patients. In addition, the system provides data on the efficacy of TSOACs, leading to improved patient care and, ultimately, cost savings surrounding adverse events.
Fulfill CMS Meaningful Use Stages 2 & 3: Provides ability to fulfill CMS MU-2 criteria for quality of Warfarin dosing at a system level with TTR > 70 percent. Achieves MU-3 measures by improving population health, provides decision support for high priority health conditions, and provides patient self-management tools via a mobile patient engagement application.
Point of Care Decision Support provides any healthcare organization a free, customized demonstration and business case report for its anticoagulant patient population.
For more information: www.ptofcare.com


 January 29, 2026
January 29, 2026 









