June 27, 2016 — The Society of Nuclear Medicine and Molecular Imaging (SNMMI) has been named a qualified provider-led entity (PLE) under the Medicare Appropriate Use Criteria program for advanced diagnostic imaging. This will allow referring physicians to use SNMMI’s appropriate use criteria (AUC) to fulfill the requirements of the 2014 Protecting Access to Medicare Act (PAMA).
PAMA requires referring physicians to consult appropriate use criteria developed by a PLE in order to ensure cost-effective and appropriate utilization of advanced diagnostic imaging services (ADIS). The legislation requires the delivery of these criteria via a clinical decision support tool, which referring physicians would need to utilize when ordering ADIS. The AUC requirements were originally due to launch by January 2017; however, the Centers for Medicare and Medicaid Services is now expected to publish more substantive information on the development of clinical decision support tools, which will delay the implementation deadline by at least a year.
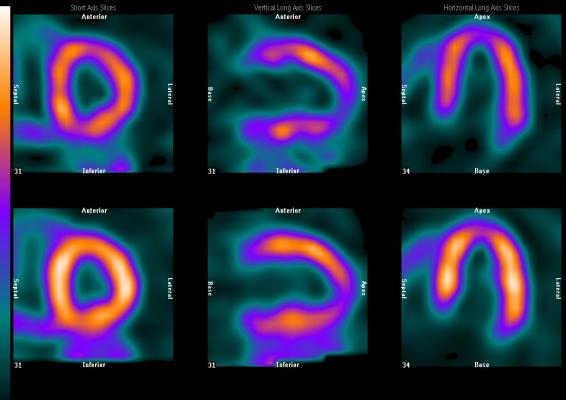
SNMMI is developing a comprehensive library of multidisciplinary, evidence-based AUC for high-value nuclear medicine procedures. It is currently finishing development of AUC for bone scintigraphy in malignant disease, ventilation perfusion imaging in pulmonary embolism, hepatobiliary scintigraphy in abdominal pain and positron emission tomography (PET)/computed tomography (CT) in restaging of malignant diseases. Development of six additional AUC has been started, including prostate cancer imaging, myocardial perfusion imaging with PET, somatostatin imaging, infection imaging, nuclear medicine procedures for thyroid cancer and gastrointestinal transit.
For more information: www.snmmi.org


 May 23, 2025
May 23, 2025