September 15, 2014 — A new minimally invasive surgery involving a stent graft made from a 3-D image of the patient’s anatomy could eliminate the need for open surgery for some patients suffering from abdominal aortic aneurysms (AAA).
An abdominal aortic aneurysm is an enlarged area in the lower part of the aorta. Since the aorta is the main supplier of blood to the rest of the body, a ruptured abdominal aortic aneurysm can be fatal. Symptoms might include belly pain or discomfort; pain in the chest, lower back or flank (over the kidneys), possibly spreading to the groin, buttocks or legs; and a pulsating sensation in the abdomen. The pain may last for hours or days. In about 10 percent of these patients, surgery is complicated because the position of the defect in the aorta is extremely close to the arteries that go into the kidneys.
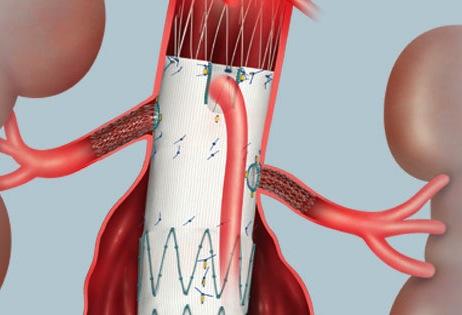
The new Zenith Fenestrated AAA endovascular graft by Cook Medical is custom-made based on the patient’s spinal CT (computed tomography) scan. From the scan, the surgeon measures the position of the vessels and where they are feeding into the kidneys, liver and intestines. Once the location is determined, the company makes the graft with a few holes or “fenestrations” on the stent graft that are designed to ensure blood flow from the aorta to the kidneys and other organs continue.
“In the past, we would have to open the belly and sometimes the chest to do this operation because it’s very complex,” said Carlos Bechara, M.D., a vascular surgeon with the Houston Methodist DeBakey Heart & Vascular Center. “For this surgery we go through the groin with no incisions and place the stent to cover the aneurysm, and then bridge the holes with stents to treat the aneurysm and at the same time preserve blood flow to these vital organs.”
Bechara says most patients discharge a few days after the procedure with minimal pain or discomfort. Results have shown less blood loss, a shorter ICU stay and a quicker return to a normal diet and regular activities than patients who undergo an open procedure to fix this problem.
“In the past, patients with abdominal aneurysms so close to the renal arteries had very limited surgical options,” Bechara said. “This new device gives us a new avenue to help these patients get back on their feet again.”
For more information: www.houstonmethodist.org


 April 26, 2023
April 26, 2023 









