News | May 11, 2015
Twelve Late-Breaking Clinical Trials to be Presented at Heart Rhythm 2015
Key studies that will help advance electrophysiology at HRS
May 11, 2015 — The Heart Rhythm Society (HRS) has released the list of its 12 late-breaking electrophysiology (EP) clinical trials that will be presented at Heart Rhythm 2015, which takes place May 13-16, 2015 in Boston.
Session I: Thursday, May 14, 1:30– 3 p.m. EDT in Ballroom West?
• Impact of Remote Monitoring on Clinical Events and Healthcare Utilization: A nationwide assessment
• VENTURE-AF: A Randomized Global Trial Evaluating Uninterrupted Rivaroxaban and Vitamin K Antagonists in Patients Undergoing Catheter Ablation for Nonvalvular Atrial Fibrillation
• Cryoballoon Versus Open Irrigated Radiofrequency Ablation in Patients with Paroxysmal Atrial Fibrillation: The prospective, randomized controlled, non-inferiority FreezeAF Study
• Botulinum Toxin Injection in Epicardial Fat Pads for Prevention of Atrial Fibrillation After Cardiac Surgery: One year follow up of randomized pilot study
• Wireless LV Endocardial Stimulation for CRT: Primary results of the Safety and Performance of Electrodes implanted in the Left Ventricle (SELECT-LV) Study
• The Evera MRI Study
Session II: Friday, May 15, 2015, 8–9:30 a.m. EDT in Ballroom West?
• Performance of a Miniaturized Transcatheter Pacing System: First-in-human experience
• Baroreflex Activation Therapy for the Treatment of Heart Failure With a Reduced Ejection Fraction: Safety and Efficacy in Patients with and without Chronic Resynchronization Therapy
• Canadian Registry of Cardiac Implantable Electronic Device Outcomes: Phase 1 Riata Lead on Advisory (CREDO)
• Clinical Safety of the Iforia ProMRI ICD System Subjected to 1.5T Cardiac or Thoracic Spine MRI
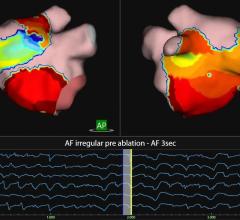
• Effect of linear ablation in substrate-based AF: Results of the Substrate Modification with Ablation and Antiarrhythmic Drugs in Nonpermanent Atrial Fibrillation trial
• Visually-Guided Laser Balloon (VGLB) Pulmonary Vein Isolation For The Treatment of Paroxysmal Atrial Fibrillation (PAF): Results Of The Prospective Multicenter Randomized Heartlight Study
For more information: www.HRSonline.org
?


 September 05, 2025
September 05, 2025