November 20, 2021 – Philips announced it signed an agreement to acquire Cardiologs, a France-based medical technology ...
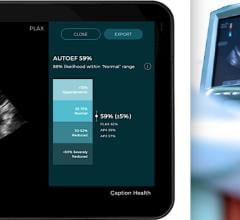
November 9, 2021 — Caption Health and Ultromics, developers of artificial intelligence (AI) to improve cardiac ...
November 9, 2021 — The COVID-19 (SARS-CoV-2) virus does not infect blood vessels, despite the high risk of blood clots ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
November 9 2021 — A new large-scale, real-world analysis of Centers for Medicare and Medicaid Services (CMS) outcomes ...
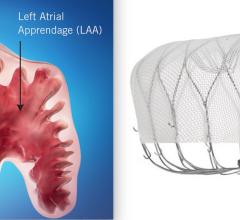
November 8, 2021 – SWISS-APERO is the first randomized clinical trial comparing the Abbott Amulet left atrial appendage ...
November 8, 2021 — Results from a clinical trial of the Edwards Lifesciences Evoque transcatheter tricuspid valve ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
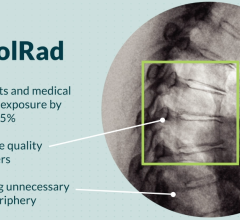
November 5, 2021 – An FDA-approved device used during cardiac cath lab procedures cut radiation exposure for ...
November 5, 2021 — In the worldwide effort to battle COVID-19 (SARS-CoV-2), researchers have often turned to medications ...

November 5, 2021 — The U.S. Food and Drug Administration (FDA) authorized the emergency use of the Pfizer-BioNTech COVID ...
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...

November 4, 2021 — The Centers for Medicare and Medicaid Services (CMS) today issued an emergency regulation now ...
November 2, 2021 — Cardionomic Inc. announce initial U.S. enrollment in its global Cardiac Pulmonary Nerve Stimulation ...
November 2, 2021 — Datascope/Getinge/Maquet is recalling its Cardiosave Hybrid/Rescue Intra-Aortic Balloon Pumps (IABP) ...
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...
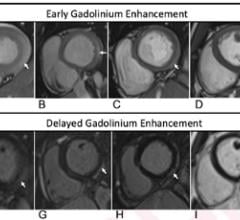
November 1, 2021 — According to ARRS’ American Journal of Roentgenology (AJR), radiologists need to be cognizant of the ...

November 1, 2021 — Here is the list of the most popular content on the Diagnostic and Interventional Cardiology (DAIC) ...
November 1, 2021 — Health AI company Vagus.co, based in Cambridge, U.K., has launched a 30-second breathing test for ...


 November 09, 2021
November 09, 2021






![Figures from an initial study on the Cardionomic CPNS technology in a poster presentation at the Heart Rhythm Society (HRS) 2021 meeting.[1] Starting at top left, an angiographic view of the atrial transseptal access for a left ventricular septal ablation. Procedural intra-cardiac echo (ICE) showing ablation catheter positioning. A 3D electro-anatomic map of the LV septum. The final graphs show baseline and post-procedure LVOT pressure readings demonstrating a decreased gradient.](/sites/default/files/styles/content_feed_medium/public/Cardionomic_Cardiac_Pulmonary_Nerve_Stimulation_CPNS_for_HF.jpg?itok=ecrU9hmQ)