April 11, 2018 — Abbott today announced the initiation of a clinical trial evaluating long-term outcomes of patients who undergo stent implantation guided by optical coherence tomography (OCT) compared to angiography. The trial (ILUMIEN IV) is the first large-scale randomized global study using Abbott's OCT imaging in patients with high-risk, complex coronary artery disease. Patients in the study will be randomized to either OCT-guided or traditional angiography to guide placement of one or more Xience everolimus-eluting coronary stents.
The first patient was enrolled by Franco Fabbiocchi, M.D., director of Invasive Cardiology Unit IV at IRCCS Centro Cardiologico Monzino in Milan, Italy.
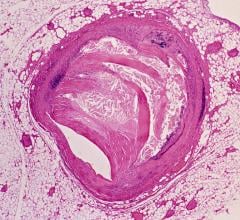
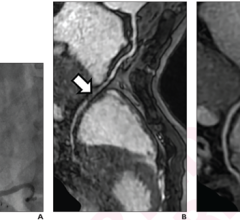
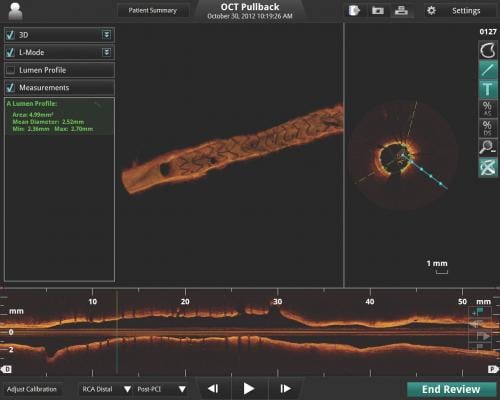
During stent implantation guided by one of Abbott's OCT platforms, physicians use high-resolution images taken directly inside the patient's vessels to accurately measure dimension and choose a stent that best fits the vessel. OCT is also used to help physicians ensure the stent is fully expanded and is flush against a vessel wall, which are both important factors in reducing stent failure.1,2
The ILUMIEN IV trial will enroll up to 3,650 patients with high-risk, complex disease at 125 centers in North America, Europe and Asia. The trial will determine if OCT-guided stent procedures result in larger vessel diameters — thus, allowing increased blood flow — and whether this will improve clinical outcomes for patients compared to stent procedures guided by angiography. Patients with complex disease may have multiple, or totally blocked arteries, or other diseases such as diabetes; and these patients account for an increasing number of cases.
"Today, most of the world uses angiography for stent implantation using a two-dimensional view of the coronary artery to assess a complex three-dimensional structure. Physicians need new technology to help optimize percutaneous coronary intervention, and OCT provides just that, the ability to look at the artery from the outside-in and the inside-out," said Ziad A. Ali, M.D., director of intravascular imaging and physiology at Columbia University Medical Center's Center for Interventional Vascular Therapy and co-principal investigator of the study. "I'm confident this technology will have a positive impact on clinical practice around the world and we hope to provide evidence for leading medical organizations to update clinical guidelines for stent implantation based on the results of this study."
The ILUMIEN IV trial's focus on high-risk patients will build on findings from the previous ILUMIEN series of trials. These showed stent procedures using OCT imaging resulted in superior stent expansion and greater rates of procedural success compared to angiography, and non-inferiority to intravascular ultrasound (IVUS) in post-procedure minimal stent area (MSA).3 Those trials also showed that use of the OCT high-resolution imaging enabled physicians to better detect damage to artery walls, called dissection, which sometimes happens during the placement of a stent compared to IVUS or angiography, which could then be repaired as necessary.4
For more information: www.abbott.com
References
1. Mintz GS, Weissman NJ. Intravascular ultrasound in the drug-eluting stent era. J Am Coll Cardiol. 2006 Aug 1; 48(3):421-9.
2. Cook S, et al. Incomplete stent apposition and very late stent thrombosis after drug-eluting stent implantation. Circulation. 2007 May 8; 115(18):2426-34.
3. Decision Resources Group, July 2017. Data on file at Abbott.
4. Ali, Z. (2016, October). Optical Coherence Tomography Compared to Intravascular Ultrasound and Angiography to Guide Coronary Stent Implantation. The ILUMIEN III: OPTIMIZE PCI trial. Presented at TCT 2016, Washington D.C.


 October 24, 2025
October 24, 2025