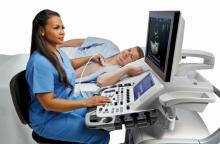
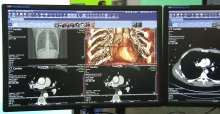
Cardiac ultrasound technology has advanced to keep up with several trends. These include improved workflow for greater efficiency, expanded use of qualification metrics, expanded use of 3-D echo to speed exam times and improve operator reproducibility, and expanded use of 3-D transesophageal echo (TEE) to aid guidance in the growing area of transcatheter structural heart procedures. Here are a few examples of how the newest technology is addressing these trends.
If you enjoy this content, please share it with a colleague
- Read more about Trends and Advances in Cardiac Ultrasound
- Log in or register to post comments