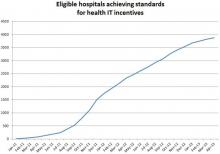
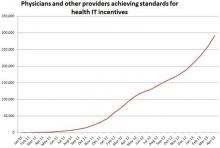
Many providers, and even consumers, are now aware of low-dose technologies, such as computed tomography (CT) systems that enable high-quality images with low radiation. Today, a growing number of hospitals are also adopting monitoring technology that takes dose management to the next level.
© Copyright Wainscot Media. All Rights Reserved.
Subscribe Now