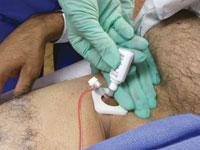
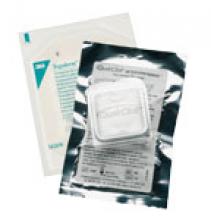
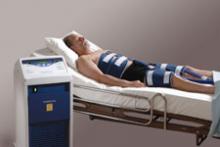
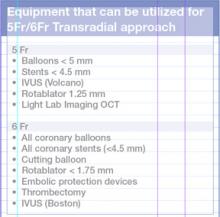
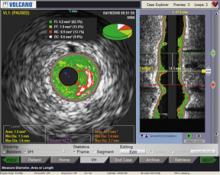
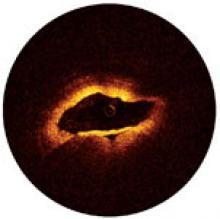
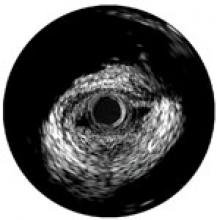
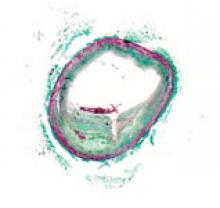
For decades, cath lab clinicians essentially had only one option for achieving hemostasis management following a procedure – compression. That changed in the 1990s with the introduction of vascular closure devices and hemostatic pads, which were designed to significantly shorten the time it took to close the wound left at the access site.
If you enjoy this content, please share it with a colleague
- Read more about Sealing the Access Site
- Log in or register to post comments