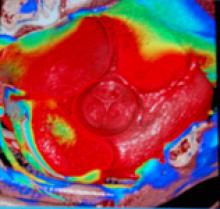
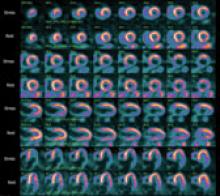
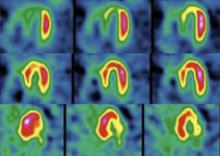
December 9, 2010 – A cardiovascular image and data management system (CIIMS) has been successfully implemented at nine Iowa Health System (IHS) affiliate hospitals. HIS used the enterprise-wide Lumedx CardioInventory system.
© Copyright Wainscot Media. All Rights Reserved.
Subscribe Now