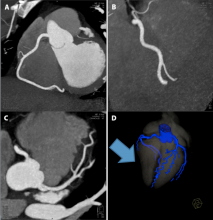
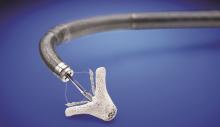
April 3, 2019 — Essential Medical Inc. received U.S. Food and Drug Administration (FDA) clearance for its large bore Manta Vascular Closure Device. It is designed to close large puncture sites in the femoral artery from larger sized transcatheter devices, including the Impella heart pump, endovascular stent grafts and transcatheter valves.
© Copyright Wainscot Media. All Rights Reserved.
Subscribe Now