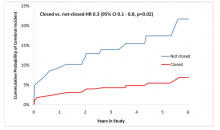
The U.S. Food and Drug Administration (FDA) announced it would hold a meeting of the Medical Imaging Drugs Advisory Committee (MIDAC) on Sept. 8 to discuss regulatory approaches for use of gadolinium-based contrast agents (GBCAs).
© Copyright Wainscot Media. All Rights Reserved.
Subscribe Now