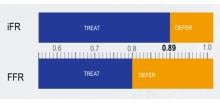
March 20, 2017 — Teleflex Inc. recently announced 510(k) clearance by the U.S. Food and Drug Administration (FDA) and U.S. commercial launch of the Spectre Guidewire.
The Spectre Guidewire is engineered with a smooth stainless steel-to-nitinol dual-core transition that balances strength and agility. It is a 0.014-inch guidewire available in 190 cm and 300 cm lengths with a distal hydrophilic coating and a proximal PTFE coating.