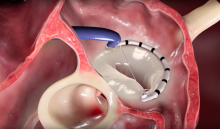
The capsule endoscopy systems offer a hands-free and highly accurate imaging modality for healthcare practitioners. A pill-sized capsule houses a miniature camera that captures the condition of esophageal tract lining. The technology was first introduced for commercial healthcare services in 2000. These capsule devices are now being used to help assess gastrointestinal issues related to cardiovascular devices.
© Copyright Wainscot Media. All Rights Reserved.
Subscribe Now