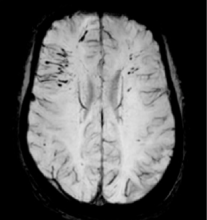
November 7, 2016 – A multicenter randomized trial evaluating the role of embolic protection using the Sentinel device during transcatheter aortic valve replacement (TAVR) found that the device was safe but did not meet the primary efficacy endpoint of reduction in median new lesion volume in protected territories assessed by MRI at two to seven days. In addition, neurocognitive function was not significantly improved.
© Copyright Wainscot Media. All Rights Reserved.
Subscribe Now