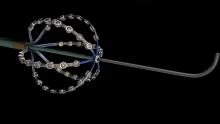
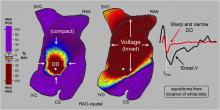
A preliminary study of a new electrophysiology (EP) mapping system using a specialized intracardiac ultrasound basket catheter for electro-mapping showed it is four times higher resolution than standard voltage mapping. Researchers also said the new mapping modality represents the electromapping data on computed tomography (CT) quality anatomy. The data suggest it may open the possibility to map irregular arrhythmias with more precision. The data was presented at Heart Rhythm Society’s 2015 meeting.
© Copyright Wainscot Media. All Rights Reserved.
Subscribe Now