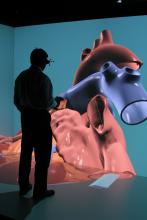
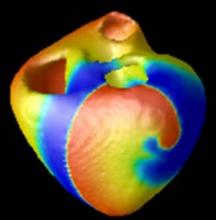
The cost of bringing innovative new cardiovascular technology to the U.S. market today is staggering, numbering in the tens of millions up to $1 billion. Many in the medical device and drug industries see this as a major barrier to developing new therapies. The majority of this cost is in the preclinical animal testing stage and the human clinical trials required to gain regulatory clearance. New technologies may very soon offer a way to greatly decrease these costs. In addition, these technologies may be able to identify which drugs or device therapies will be effective in humans before spending millions in potentially dead-end research.
If you enjoy this content, please share it with a colleague
- Read more about Cardiovascular Clinical Trials of the Future
- Log in or register to post comments