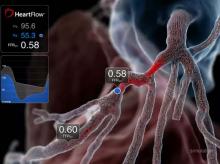
Adding the HeartFlow FFRCT Analysis to a standard coronary computed tomography angiogram (cCTA) may change the course of treatment in more than one-third of patients with coronary artery disease. This conclusion was discussed in a study presented at the EuroPCR 2015 conference.
© Copyright Wainscot Media. All Rights Reserved.
Subscribe Now