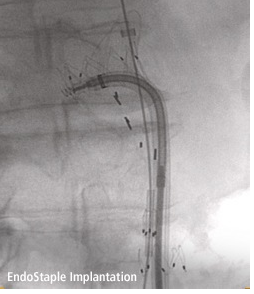
May 24, 2011 — Aptus Endosystems Inc., a medical device company developing advanced technology for endovascular aneurysm repair (EVAR), announced CE Mark approval for the Aptus EndoStapling System. The innovative helical staple technology enables independent endograft fixation and is designed to mimic the hand suturing performed during open surgical repair of abdominal aortic aneurysms. Aortic aneurysms are an enlarged and weakened section of the aorta, which can be lethal if left untreated. Each year, an estimated 100,000 people in Europe and about 200,000 people in the United States are diagnosed with this condition. In EVAR, an alternative to open surgical repair, a metal and fabric endograft is implanted using a catheter-based delivery system to isolate blood flow away from the aneurysm to prevent potential rupture and death.
The EndoStapling System provides physicians with a novel technology to repair endovascular grafts that have exhibited migration or endoleaks (or are at risk of these complications, which are commonly seen after EVAR), where augmented radial fixation and/or sealing is required to regain or maintain effective aortic aneurysm exclusion. The system also can be used during de novo EVAR procedures (at the time of initial endograft implantation) to enhance an endograft’s inherent fixation and sealing mechanisms.
“The use of the Aptus EndoStapling System will be a great advantage to simplify revision surgery for migrated endografts,” said Jean Paul de Vries, M.D., Ph.D., Head of the Department of Vascular Surgery, St. Antonius Hospital, Nieuwegein, The Netherlands. “The ability to staple migrated endografts to the aortic wall and to fixate extension cuffs will overcome most migrations and type IA endoleaks. This system truly mimics a sutured anastomosis.”
In addition, de Vries said, “EndoStaples can improve fixation of de novo endografts in challenging proximal necks – short, large and angulated – and can potentially reduce distal migration during followup.”
“We found the system to be a highly sophisticated, user-friendly device, designed to be applied easily by the average user, requiring a short learning curve,” said Theodossios Perdikides, M.D., Director of Vascular Surgery, Hellenic Air Force Hospital, Athens, Greece. “I believe this innovative technology promises to prevent or repair major EVAR-related pitfalls that can lead to failure or even disaster.”
“This is a big leap forward concerning minimally invasive bail-out for endografts at risk for migration,” said Joerg Tessarek, M.D., chief of vascular surgery, St. Bonifatius Hospital, Lingen, Germany. “Not only for traditional infrarenal devices, but also for complex fenestrated or branched grafts where migration affects target vessel patency, this will be a helpful tool to provide graft stability.”
In addition to use with the Aptus endograft (which received CE Mark approval in 2009), the Aptus EndoStapling System has received CE approval for use with the Cook Zenith, Gore Excluder and Medtronic AneuRx, Endurant and Talent endografts in both initial implant and secondary repair settings. Use with other endografts has not been evaluated.
For more information: www.aptusendosystems.com


 April 26, 2023
April 26, 2023 









