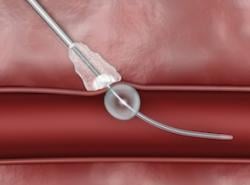
November 6, 2014 — Cardinal Health announced that its MynxGrip Vascular Closure Device recently received U.S. Food and Drug Administration (FDA) approval for use to close femoral veins. The MynxGrip device is now indicated for use to seal 5, 6 and 7 French femoral arterial and femoral venous access sites.
The venous indication could help interventional healthcare providers increase efficiency of their labs and minimize potential complications associated with venous closure by replacing the need for manual compression. The MynxGrip device is intended to reduce times to hemostasis and ambulation, thereby potentially shortening post-procedure recovery times.
The MynxGrip Vascular Closure Device utilizes the proprietary, extravascular Grip sealant that actively adheres to the vein for a secure mechanical closure and dissolves within 30 days, leaving nothing permanently behind in the healed vein. The safety profile of this secure extravascular sealant makes the MynxGrip device uniquely suited for venous closure, as it does not leave behind an intravascular component.
"The gentle deployment and secure extravascular sealant make the MynxGrip device an excellent option for closing femoral veins,” said Sanjay Srivatsa, M.D., director, Heart Artery and Vein Center, Fresno, Calif. “I feel confident that this indication will change the way interventionalists and electrophysiologists approach venous access sites, enabling them to close their more complex cases on the table, and thereby helping to increase efficiency and throughput in busy interventional laboratories."
The new venous indication does not include any changes to the existing MynxGrip Vascular Closing Device. Cardinal Health is mailing new instructions for use documents and patient brochures to all current MynxGrip users and those users can immediately begin using the existing device to close femoral veins.
For more information: www.accessclosure.com


 October 07, 2025
October 07, 2025 









