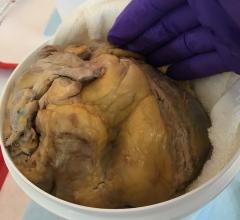
March 3, 2014 — The U.S. Food and Drug Administration (FDA) cleared Admedus’s CardioCel, a tissue product to repair and treat a range of cardiovascular and vascular defects. The company is looking to complement its existing product launch in Europe with preparation for initial sales in the United States.
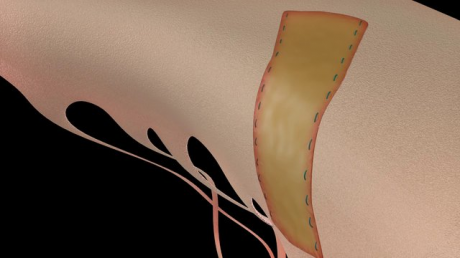
The intended use of CardioCel in the United States is in pericardial closure and for the repair of cardiac and vascular defects in both adults and paediatrics.
Admedus can sell CardioCel in both Europe and the US and will pursue market approvals in Asia and other jurisdictions.
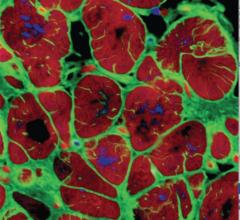
CardioCel is engineered by the group’s ADAPT tissue engineering process. It intends to be a durable, pure collagen scaffold that avoids calcification, supports native cell infiltration, growth and differentiation and which promotes a regenerative healing process.
CardioCel has shown that it does not calcify and can facilitate autologous tissue regeneration once surgically implanted, while retaining strength and natural elasticity. CardioCel is a ready to use, off the shelf product that has the potential to prevent follow up surgeries for patients because of its anti-calcification and regenerative properties.
For more information: www.admedus.com


 November 19, 2021
November 19, 2021