March 24, 2009 - W. L. Gore & Associates today said it received FDA approval to market a 31 mm diameter version of the GORE EXCLUDER AAA Endoprosthesis.
The company said the device provides physicians with a safe and effective endovascular option to treat abdominal aortic aneurysms (AAA) in patients with aortic inner neck diameters up to 29 mm. Other important enhancements include a flat-top design that enables greater production efficiency, an additional pair of proximal anchors to help ensure excellent fixation, and a lengthening of the trunk from 7 to 8 cm to accommodate larger anatomies.
More than 84,000 GORE EXCLUDER Devices have been implanted in patients worldwide, making it a widely accepted, minimally invasive treatment option for individuals with AAA. The 31 mm device will be available for clinical use in the U.S. in May 2009. It has been available outside of the U.S. since 2004 and has been implanted in more than 3,300 patients.
AAA involves swelling or bulging of the abdominal aorta. When the condition is not treated it can lead to aortic rupture, which is often fatal. Traditional surgical treatment requires a large incision to place a synthetic graft to repair the diseased artery. This method can result in long hospital stays and painful recoveries. The GORE EXCLUDER AAA Endoprosthesis, in contrast, uses a minimally invasive procedure, thereby reducing the hospital stay, morbidity and mortality associated with surgery.
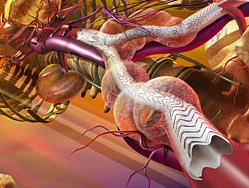
The Gore device is an endovascular graft and stent combination that seals off the aneurysm and creates a new path for blood flow. The device is inserted through a small incision in the patient’s leg using a catheter-based delivery technique. Once the physician has positioned the graft in the diseased aorta, the device self-expands using radial force. Metal anchors help secure it to the walls of the aorta.
The 31 mm GORE EXCLUDER Device will be available in 13, 15 and 17 cm lengths. A new 32 mm x 4.5 cm Aortic Extender Component will join the device family as well. Both the new Trunk-Ipsilateral Leg and Aortic Extender Components are 20 Fr. introducer sheath compatible.
For more information: www.goremedical.com


 April 26, 2023
April 26, 2023 









